Article Text
Abstract
Background/hypothesis Shoulder pain in elite swimmers is common, and its pathogenesis is uncertain.
Hypothesis/study design The authors used a crosssectional study design to test Jobe’s hypothesis that repetitive forceful swimming leads to shoulder laxity, which in turn leads to impingement pain.
Methods Eighty young elite swimmers (13–25 years of age) completed questionnaires on their swimming training, pain and shoulder function. They were given a standardised clinical shoulder examination, and tested for glenohumeral joint laxity using a non-invasive electronic laxometer. 52/80 swimmers also attended for shoulder MRI.
Results 73/80 (91%) swimmers reported shoulder pain. Most (84%) had a positive impingement sign, and 69% of those examined with MRI had supraspinatus tendinopathy. The impingement sign and MRIdetermined supraspinatus tendinopathy correlated strongly (rs=0.49, p<0.00001). Increased tendon thickness correlated with supraspinatus tendinopathy (rs=0.37, p<0.01). Laxity correlated weakly with impingement pain (rs=0.23, p<0.05) and was not associated with supraspinatus tendinopathy (rs=0.14, p=0.32). The number of hours swum/week (rs=0.39, p<0.005) and weekly mileage (rs=0.34, p=0.01) both correlated significantly with supraspinatus tendinopathy. Swimming stroke preference did not.
Conclusions These data indicate: (1) supraspinatus tendinopathy is the major cause of shoulder pain in elite swimmers; (2) this tendinopathy is induced by large amounts of swimming training; and (3) shoulder laxity per se has only a minimal association with shoulder impingement in elite swimmers. These findings are consistent with animal and tissue culture findings which support an alternate hypothesis: the intensity and duration of load to tendon fibres and cells cause tendinopathy, impingement and shoulder pain.
Statistics from Altmetric.com
Introduction
Millions of people participate in and enjoy aquatic sports, mostly swimming for fitness or competition. Sports injury surveys confirm that recreational swimmers have a low injury potential because of the buoyant effect of water.1 However, as in any competitive sport, injury and pain can affect the elite swimmer. Swimming training involves repetitive overhead movement. In all the main swimming strokes (freestyle, backstroke, breaststroke and butterfly), the swimmer uses large moment arm forces to reach forward to drag the water. Where the training is intense, these factors may all contribute to shoulder injury and pain.2,–,5 Competitive swimmers begin their careers as early as age seven, and most of them train and take part in year-round competitions. Competitive swimmers typically complete 2500 or more shoulder revolutions per day.6
The condition of ‘swimmer’s shoulder’ was first described by Kennedy and Hawkins7 in 1974 as a ‘common, painful syndrome of repeated shoulder impingement in swimmers’. Many studies have reported shoulder pain in elite swimmers.3 7,–,16 The prevalence of shoulder pain in swimmers was 3% in a study published in 19747 and has increased in recent publications: 42% in 1980,16 68% in 1987,13 73% in 1993,3 40–69% in 199417 and 5–65% in 1996.18
Proposed causes of swimmer’s shoulder
A clear consensus is lacking as to the cause(s) of shoulder pain in swimmers. Suggestions have been made that swimmer’s shoulder represents a part of the impingement syndrome complex, rotator cuff tendonitis, biceps tendonitis and shoulder instability.15 19,–,21 Other pathological shoulder conditions, such as labral tears and acromioclavicular joint disruption, have also been observed in swimmers.22,–,24
As swimmers engage in repetitive overhead motion, it is possible that swimmer’s shoulder may involve glenohumeral joint laxity and the ‘instability complex’ described by Jobe et al.25–26 Jobe et al25 hypothesised that repetitive and forceful overhead activity causes a gradual stretching out of the anteroinferior capsuloligamentous structures leading to mild laxity, instability and impingement. Figure 1 summarises their hypothesis.
This hypothesis has been quoted extensively and has been used to design protocols to treat swimmers and other overhead athletes with shoulder pain. However, on review of the literature, there is little information that supports or refutes this hypothesis.
Laxity measurement
The normal range of glenohumeral joint laxity is unknown.27 Excessive translation of the humeral head on the glenoid is normally prevented during athletic activities by static stabilisers of the glenohumeral joint, especially the glenoid labrum and capsular ligaments. Repetitive stretch injury or traumatic injury to the capsular ligaments may adversely affect static stabilisation and increase the risk of instability to the joint. Instability has been defined as a symptom secondary to increased laxity.28
Many clinical tests have been described for assessing shoulder laxity and instability.29,–,32 However, most of these tests are subjective, do not provide a continuous scale of numeric values and have poor inter- and intraobserver reliability.31 Neer and Foster32 originally described the sulcus sign test as a test for multidirectional instability. A sulcus sign is a palpable and visible dimple created beneath the acromion when applying an inferior force, pulling downward on the subject’s arm while in a sitting position.29 32 Tzannes and Murrell studied multidirectional instability which was defined as the presence of grade 2 or greater laxity in more than one direction, in a symptomatic shoulder, on examination under anaesthesia. They confirmed that the sulcus sign is a good indicator of multidirectional instability.30 33 However, when performing this test, the inferior force applied is not standardised, and assessment of the dimple size is subjective. With this manual assessment, interobserver reliability is only fair (intraclass correlation coefficient (ICC)=0.60).33
Electronic quantification of translation in the knee joint, using the KT-1000,34 is well established. However, there have been few attempts to quantify shoulder laxity with instrumentation. Jørgensen and Bak35 have applied the use of a Donjoy knee laxity tester (dj Orthopaedics, Vista, California) to study anteroposterior translation of the glenohumeral joint. Sauers et al36 developed an instrumented arthrometer to assess glenohumeral joint laxity anteriorly and posteriorly at four force levels. As noted by Tibone et al,37 it has been very difficult to find a clinical device that provides an easy-to-use, objective, accurate, non-invasive and reproducible measurement of shoulder laxity. We recently described an instrumental shoulder laxometer with these features that can measure inferior translation of the glenohumeral joint.29 In the present study, we used this laxometer to test the laxity of shoulders in elite swimmers.
Supraspinatus tendinopathy
Supraspinatus tendinopathy (ie, supraspinatus tendinosis or tendinitis) is another candidate cause of swimmer’s shoulder. The supraspinatus is the major rotator cuff muscle responsible for securing the humeral head in the glenoid, and its tendon is susceptible to tendinopathy in swimming and other overhead sports.4 5 21 25 38 39 The normal tendon appears yellowwhite. When magnified, quiescent rows of tenocytes (tendon cells of fibroblast origin) can be seen interspersed among the compact parallel bundles of collagen fibres which in turn comprise collagen fibrils. In supraspinatus tendinopathy, the tendon usually changes from yellow-white to grey, becoming dull and oedematous, often with the bursa appearing inflamed. Microscopically, the tissue appears disrupted. Although there is little evidence of inflammatory cells, the tissue appears hypercellular with fibroblastic cells in varying states of cell health.39
The aim of this study, therefore, was to investigate the pathogenesis of swimmer’s shoulder. To do this, we examined (1) which clinical sign best predicts shoulder pain in swimmers; (2) which anatomical structure(s) is/are associated with this shoulder pain; (3) whether repetitive swimming movements are associated with increased shoulder laxity; (4) whether increased laxity is associated with impingement pain; and (5) the extent, if any, to which training characteristics are associated with supraspinatus tendinopathy, laxity or both in elite swimmers.
Method
Swimmer recruitment
Under ethics approval, 86 elite swimmers were recruited from four competitive swimming clubs. All swimmers met the following inclusion criteria: (1) aged between 13 and 25 years; (2) having trained with a coach for a minimum of 2.5 years; and (3) swimming for at least 6 h/week. Exclusion criteria were: (1) previous surgery on the involved shoulder; (2) previous fracture of the shoulder; and (3) inability or unwillingness to participate in the MRI and clinical shoulder examination. Six elite swimmers were excluded from the study. Two were older than the age inclusion criterion, one had a dislocated shoulder, one inadvertently enrolled twice, and two failed to sign the consent form, leaving a total of 80 swimmers for the study.
Swimming training
Each study participant administered swimming requested the following information: number of years with coaching; hours per week in swimming training; level of competition (international, national, state or club level); weekly swimming distance; personal best in highest ranked event; and percentage of time in training spent in each stroke over the previous 3 months.
Shoulder pain and function
Descriptive characteristics were obtained for each swimmer using a standardised Shoulder Service Questionnaire, which is a modification of a validated questionnaire.40 Items pertaining to the subject included age, gender, birth date, occupation, arm dominance (right, left or ambidextrous) and an overview of general health. Clinical parameters of the shoulder condition included affected shoulder (right or left), date of injury onset, mechanism of onset (whether traumatic or insidious), presence of an insurance claim, level of activity at work and highest exercise level before shoulder problem. All items contained in the Shoulder Service Questionnaire were scored on a five-point rating scale (0–4) except for the current level of activity, which was scored on a four-point rating scale (1–4). This questionnaire was designed to indicate the frequency and severity of the shoulder pain (with activity, at night and at rest), stiffness of the shoulder, difficulty in reaching behind the back, difficulty with activities above the head, overall shoulder status (very bad, bad, fair, good), current level of activity (none, light activity, moderate activity and strenuous labour) and highest level of sport at the time of examination (none, hobby sport, club sport, state sport, national and international sport).
Clinical examination
Clinical shoulder examinations were performed on all 80 subjects. For the analysis, data from one shoulder of each subject were included. The shoulder chosen was the affected shoulder if symptomatic or the dominant shoulder if asymptomatic. Each athlete was given a systematic clinical shoulder examination which included 23 clinical tests. The results of each of these tests were recorded on a standardised Examiner’s Form. The validity, reliability and clinical usefulness of the majority of these tests have been previously described.15 25 33 41,–,45
Laxometer
We used a non-invasive electronic device for testing inferior translation of the humeral head in humans which has been shown to have excellent reliability and validity, that is, patients with shoulder instability have higher laxity readings.29 All subjects in this study were tested with the laxometer for inferior translation of their glenohumeral joint.
Magnetic resonance imaging
Fifty-three swimmers were available for MRI. One was unable to tolerate more than two pulse sequences and was excluded from the MRI study, leaving 52 swimmers for this aspect of the study.
MRI was used in a systematic fashion by a single examiner trained in musculoskeletal MRI (JL) using a standardised form to determine the minimum thickness of the supraspinatus tendon and to assess for acromion shape, tendinopathy, tears, acromioclavicular joint arthritis and other pathological conditions in the shoulder. Minimum tendon thickness was measured immediately medial to the insertion of the supraspinatus tendon into the greater tuberosity of the humerus, 1 cm posterior to the biceps tendon.
The swimmers’ shoulders were examined with a 1.5 T MRI system (Signa; GE Medical Systems, Milwaukee, Wisconsin) and system software 9.1 (slew rate, 77 T/m/s, 33-mm T gradient amplitude), with the use of a high-resolution, non-arthrographic technique with a four-channel phased-array shoulder coil (Medical Advances, Milwaukee, Wisconsin). Oblique coronal proton-density and fat-suppressed T2, sagittal T2 and axial proton-density sequencing were performed (table 1).
MRI shoulder protocol
MRI-assessed supraspinatus tendinopathy
Supraspinatus tendinopathy was graded using a modified four-point scale from 0 to 3.46 For grade 0 (normal), the tendon showed complete homogeneous low intensity on all pulse sequences. For grade 1 (mild tendinopathy) there was a mild focal increase in tendon signal on proton density (PD) and fat-suppressed T2 sequencing not equal to that of fluid. Grade 2 (moderate tendinopathy) represented a moderate focal increase in tendon signal on PD and fat-suppressed T2 sequencing not equal to that of fluid. For grade 3 (marked tendinopathy), the tendon showed a marked generalised increase in tendon signal without frank fluid signal intensity. The grading of MRI-determined supraspinatus tendinopathy is very reliable (ICC=0.75) when assessed by a single welltrained observer.46
Statistical analyses
Statistical tests were carried out using SigmaStat software (Systat Software, Point Richmond, California). Multiple logistic regression analysis was performed with supraspinatus tendinopathy as the dependent variable and training regime parameters as independent variables. Statistical analyses that compared laxity with training and symptoms were based on data from all 80 swimmers, whereas the MRI analyses were based on a subset of 52 swimmers.
Results
Demographics of the swimmers
The age range of the swimmers in this study was 13–25 years. The mean (±SD) age was 15.9 (±2.7) years, and the median age was 16 years. Forty-two (53%) of the elite swimmers were male, and 38 (47%) were female. Of the total participants, 13/80 represented their country at an international level, 41/80 swam at the national level, 24/80 were at the state level, and 2/80 swimmers were at the club level. With regards to the swimmers’ main event, 35% specialised in freestyle, including two open water swimmers, 19% specialised in butterfly stroke, 24% specialised in backstroke, 18% specialised in breaststroke, and 5% were individual medley swimmers. Table 2 shows the percentage of training time spent in each of the four strokes.
Elite swimming training profiles
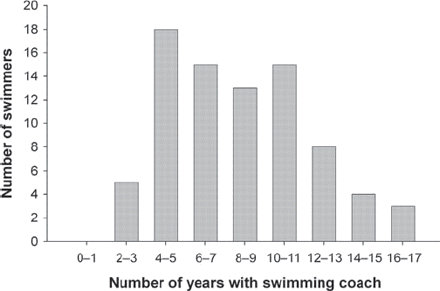
All swimmers had been coached for at least 2.5 years, ranging upwards to a maximum of 17 years, with a median time of 8 years (figure 2). According to their questionnaire responses, the amount of time the swimmers practised in the water varied between 8 and 29 h/week, and the median number of hours they spent in water training was 16 h/week (figure 3). They swam between 9 and 110 km/week with a median distance of 40 km/week (figure 4). Ninety per cent (72/80) of the swimmers spent more than 50% of their training time in freestyle. On average, they estimated that they spent approximately 13% of their swimming time in butterfly stroke, 21% in back stroke and 13% in breaststroke (figure 5).
Number of years with coaching, median 8 years.
Number of hours swum per week, median 16 h.
Number of kilometres swum per week, median 40 km.
Percentages of the total swimming training time spent with each of the four main strokes.
Shoulder pain and function
Of the total study group of 80 swimmers, 43 (54%) reported unilateral shoulder pain (affecting 28 right shoulders and 15 left shoulders), and 30 (37%) others reported bilateral shoulder pain. The remaining seven swimmers (9%) stated they had no shoulder pain. Sixty-four swimmers (80%) reported they had pain during activity, and of these, 25 had activity pain monthly, 22 had it weekly, 14 had it daily, and three stated they always had shoulder pain during activity. Fifty-six out of 80 (70%) swimmers specified that they had shoulder pain with activity above their head. Twenty-two (28%) swimmers had shoulder pain at night. Of this group, 14 swimmers had night pain monthly, six had it weekly, one had pain every night, and another stated she had pain continuously throughout the night. Twenty-two (28%) swimmers rated the level of their night pain as mild to moderate. Twenty-four out of 80 (30%) swimmers reported they had extreme pain monthly, while nine had it weekly. The same proportion of swimmers indicated they had mild to moderate shoulder pain, even while resting. Fifty-four out of 80 (68%) swimmers indicated they had shoulder stiffness. Of these, 42 (52%) had difficulty reaching behind their back, and 43 (54%) had difficulty with activities above their head. Four (5%) of the 80 swimmers had severe to very severe difficulty with overhead activities, whereas the remainder had mild to moderate difficulty.
Of the total study group, 41/80 (51%) self-assessed the overall condition of their shoulder as fair (4) on a scale of 1–5 (very bad to good). Ten out of 80 (12.5%) reported their shoulder to be in poor condition (3), and five out of 80 (6%) in bad condition (2). None of the swimmers permanently discontinued swimming because of their shoulder problem. Eighty-nine per cent had sought treatment for their shoulder pain: 16/80 (20%) with chiropractic therapy, 47/80 (59%) with physiotherapy and 9/80 (11%) with acupuncture, while 8/80 (10%) had no treatment for their pain.
Clinical examination of the shoulder
Details regarding the clinical examination findings in swimmers are shown in table 3. The most common positive findings were a positive impingement sign (90%) and signs related to the biceps and A-C joint: biceps tenderness (45%), Paxinos sign (34%), O’Brien sign (25%) and A-C joint tenderness (10%).
Clinical examination results
Glenohumeral joint laxity in swimmers
Glenohumeral joint laxity was manually assessed during the clinical examination for a sulcus sign. None of the swimmers had a grade 3+ sulcus sign. In 11 (14%) of simmers, the sulcus sign was assessed as grade 2+, and in 41 (51%) as grade 1+. Forty-nine (61%) swimmers had grade 1+, and eight (10%) had grade 2+ anterior translation. Twenty-six (33%) swimmers had grade 1+, and four (5%) had grade 2+ posterior translation.
An instrumented laxometer was also used to test inferior translation of the humerus in both shoulders of the 80 swimmers. With this technique, the range of inferior translation of the glenohumeral joint was 0.5–5.9 mm with a median value of 1.5 mm for both male and female shoulders. For the age range studied here, there was no significant correlation between swimmer age and shoulder laxity (r=0.097, p=0.49).
Within the entire group of swimmers, shoulder laxity correlated positively with a greater range of motion in internal rotation (Pearson product moment correlation, r=0.33, p<0.05). The entire group had more inferior glenohumeral joint translation in the left shoulder than in the right (paired t tests, p<0.01). Significantly more laxity was also noted in the left shoulders of females than in their right shoulders (paired t tests, p<0.05). Females had significantly more laxity than males (1.72 mm vs 1.28 mm; t test, p<0.05). However, the difference in glenohumeral joint laxity between male left and right shoulders was insignificant.
MRI findings in elite swimmers
Supraspinatus tendinopathy
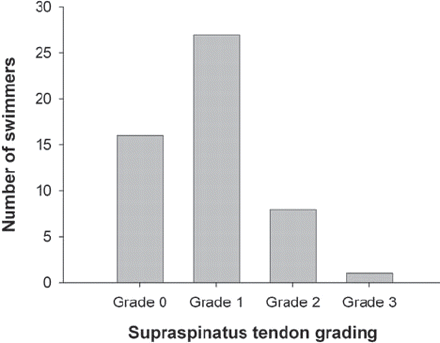
Thirty-six (25 male, 11 female) or 69% of the 52 swimmers that were imaged by MRI showed evidence of supraspinatus tendinopathy. Of those with tendinopathy, 27/36 (75%) had their tendinopathy assessed as grade 1, 8/26 (22%) as grade 2 and 1/36 (3%) as grade 3 (figure 6). Of the imaged population, 4/4 (100%) swimmers at the international level exhibited supraspinatus tendinopathy, 24/27 (89%) swimmers at the national level and 8/20 (40%) swimmers at the state level, and 0/1 (0%) swimmers at the club level had supraspinatus tendinopathy (figure 7). This condition was found in 13/24 (54%) of swimmers aged between 13 and 14 years, 10/13 (77%) between 15 and 16 years, 8/8 (100%) between 17 and 18 years and 5/7 (71%) between 19 and 22 years of age. This study found no association between preferred swimming stroke and supraspinatus tendinopathy.
MRI-determined supraspinatus tendinosis in elite swimmers.
Relationship between competition level and supraspinatus tendinosis in elite swimmers (rs=0.56, p<0.00005, using Spearman rank order correlation).
Other MRI findings
For all swimmers, MRI-determined minimum supraspinatus tendon thickness ranged from 6 to 10 mm, with a median value of 8 mm. Twenty-seven per cent of the swimmers had thickened supraspinatus tendons. There was no relationship between tendon thickening and the swimmers’ ages.
The biceps tendon was normal in 46 imaged shoulders. The biceps anchor was reported to be unstable in three swimmers’ shoulders, and three other shoulders were found to have biceps sheath effusion. Tears of the supraspinatus tendon were found in three swimmers: two were reported as having a delaminated intrasubstance tear, and one had a partial 3 mm articular side tear. Two had subscapularis tendinopathy, and one had infraspinatus tendon thickening, but no change was reported for the teres minor tendon.
Labral tears were detected in 10/52 swimmers whose shoulders were imaged. Eight swimmers had superior labrum anterior posterior (SLAP) lesions. One had a SLAP lesion and Bankart lesion, and another had a Bankart lesion alone. Mucoid changes in labrum were noted in five swimmers’ shoulders, and two were noted to have a paralabral cyst. Five sublabrumforamens and one Buford complex were also observed.
The acromion shape was assessed as type I in 20/52 (38%) swimmers, type II in 29/52 (56%) and type III in 3/52 (6%). Thickening of the subacromial bursa was reported in 33/52 (63%) swimmers, and thickness of the subscapularis bursa affected 3/52 (6%) others. Minimal to moderate increase in subacromial and subdeltoid fluid was observed in 21/52 (40%) swimmers’ shoulders, while 7/52 (13%) of the swimmers had acromioclavicular joint arthritis. In 17/52 (33%) of the swimmers’ shoulders, the acromion ossification centre had not yet fused.
Statistical correlations
Laxity correlated modestly with impingement (rs=0.28; p<0.05) but lacked significant association with supraspinatus teninopathy. There were significant relationships between laxity and extreme pain (p<0.05), and between impingment during external rotation and shoulder pain (rs=0.25, p<0.05). The correlation between laxity and pain during activity approached but did not reach significance (rs=0.21; p=0.065). There was no correlation between laxity and level of competition, hours of training or weekly mileage.
In contrast, the swimmers’ supraspinatus tendon thickness correlated significantly with their (1) level of training (r=0.28, p<0.05); (2) years in training (r=0.35, p<0.01); (3) hours per week in training (r=0.33, p<0.05); and (4) cumulative use (weekly distance×48 week×no of years with coach) (r=0.37, p<0.01). All swimmers with increased tendon thickness had impingement pain and supraspinatus tendinopathy.
All but one of the 36/52 (69%) swimmers who had supraspinatus tendinopathy indicated they had shoulder pain on a diagram included in the shoulder pain and function questionnaire. However, correlations were lacking between supraspinatus tendinopathy and severity or frequency of shoulder pain during activity, at night or at rest (p>0.05). Stepwise multiple regression analysis showed that the dependent variable ‘pain during activity’ could be predicted from a linear combination of two independent variables: impingement sign (in either direction) r2=0.16 (p<0.05) and the Paxinos sign r2=0.10 (p<0.05) in the elite swimmers.
A positive impingement sign correlated strongly with the presence of supraspinatus tendinopathy (rs=0.49, p<0.00001). A positive impingement sign had 100% sensitivity and 65% specificity for diagnosing supraspinatus tendinopathy. The presence of a positive impingement sign failed to correlate significantly with other shoulder pathologies including labral tear, biceps instability, MRI-determined acromial types (1,2,3), supraspinatus tendon thickness, bursal thickness, sublabral-foramen, acromioclavicular joint arthritis or an unfused acromion ossification centre. A significant inverse association was noted between supraspinatus tendinopathy and a positive apprehension sign (rs=?0.33, p<0.05). Apart from tests for the impingement sign and apprehension, there were no significant correlations between supraspinatus tendinopathy and any of the clinical tests (see online supplementary material).
Spearman correlation coefficients were used to test the relationships between supraspinatus tendinopathy and the swimming training (years of coaching, percentage of stroke specialty, level of competition, hours of training and weekly mileage). There was no association between supraspinatus tendinopathy and the number of years coached (p>0.05).
The swimmers stated that their percentages of swimming training time spent with each of the four main strokes were: freestyle (25–95%) with a median percentage of 50%; butterfly (0–40%) with a median percentage 11.25%; backstroke (2–70%) with a median percentage of 20%; and breaststroke (0–45%) with a median percentage of 10%. Except for inverse relationships between the percentage of swimming time spent in butterfly stroke and supraspinatus tendon thickness (rs=?0.30, p<0.05), or between supraspinatus tendinopathy (r=?0.30, p<0.05) and the number of years coached (rs=?0.29, p<0.05), there were no significant associations for freestyle, backstroke or breaststroke.
There was a highly significant association between supraspinatus tendinopathy and competition level (figure 7). Athletes at higher levels of competition were more likely to have supraspinatus tendinopathy than those at lower levels of competition (rs=0.56, p<0.0001).
A significant association was also found between supraspinatus tendinopathy and the number of hours swum/week (rs=0.36, p<0.01). Swimmers who swam more than 15 h/week were more likely to have supraspinatus tendinopathy than those who swam fewer hours, (rs=0.48, p<0.0005). All swimmers who swam more than 20 h/week had supraspinatus tendinopathy (figure 8). Elite swimmers who trained for more than 15 h/week were twice as likely to have tendinopathy as those who trained for less time (rs=0.48, p<0.0001).
Relationship between hours swum per week and supraspinatus tendinosis in elite swimmers (rs=0.39, p<0.005, using Spearman rank order correlation).
There was also a significant association between supraspinatus tendinopathy and the number of kilometres swum per week (rs=0.33, p<0.05). The swimmers who swam more than 35 km/week were more likely to have supraspinatus tendinopathy than those who swam fewer kilometres (rs=0.37, p<0.01). Importantly, all swimmers who swam more than 60 km/week exhibited supraspinatus tendinopathy (figure 9). Athletes who swam more than 35 km/week (r=0.37, p<0.01) were four times more likely to have tendinopathy than those who swam fewer kilometres.
Association between kilometres swum per week and supraspinatus tendinosis in elite swimmers (rs=0.34, p=0.01 using Spearman rank order correlation).
Stepwise multiple regression analysis was used to determine the relationship between supraspinatus tendinopathy and the significant independent variables of swimming training: the number of hours swum per week and the weekly mileage. The level of competition was omitted, since this is not a variable that the swimmers could voluntarily change. This analysis showed that the dependent variable ‘supraspinatus tendinopathy’ could be predicted from the independent variable ‘hours swum per week’ (r2=0.131, p<0.01). Multiple logistic regression analysis showed that supraspinatus tendinopathy could be correctly predicted in 85% of elite swimmers either from the number of hours swum per week alone or in combination with the swimmer’s weekly mileage. In essence, swimmers who train more than 15 h/week and swim a weekly distance of more than 35 km are at increased risk for supraspinatus tendinopathy.
Stepwise multiple regression analysis showed that the dependent variable ‘pain during activity’ in the elite swimmers could be predicted from a linear combination of two independent variables: a positive impingement sign (in either direction) r2=0.16 (p<0.05) and the Paxinos sign r2=0.10 (p<0.05). Multiple logistic regression analysis, performed with pain during activity as the dependent variable and the impingement sign and the Paxinos sign as independent variables, showed that the presence of absence of these signs could predict pain during activity in 84% of elite swimmers.
Similarly, the best predictors for pain level with activities above the head in elite swimmers were the impingement sign (r2=0.11; p<0.05) and O’Brien test (r2=0.17; p<0.05). Multiple logistic regression analysis demonstrated that the impingement sign and O’Brien’s sign could predict pain level with activities above the head in 75% of elite swimmers.
In summary, of the elite swimmers in our imaging study, 36/52 (69%) had supraspinatus tendinopathy. Each swimmer with supraspinatus tendinopathy had a positive impingement sign. The positive impingement sign correlated strongly with MRI assessment of tendinopathy. Moreover, all swimmers with supraspinatus tendon thickening had impingement and supraspinatus tendinopathy. There was a modest, yet significant, correlation between glenohumeral joint laxity and impingement pain. There were highly significant associations between supraspinatus tendinopathy and competition level, and the number of hours swum per week. Supraspinatus tendinopathy also correlated significantly with the swimmers’ weekly mileage.
Discussion
An evaluation of Jobe’s hypothesis that glenohumeral joint laxity is a cause of shoulder pain
Using manual laxity tests, several authors have examined glenohumeral joint laxity in recreational and/or competitive swimmers, and concluded that competitive swimmers have greater glenohumeral and general joint laxity.6 8 14 18 47 Borsa et al48 disagreed. Using sonographic imaging to quantify glenohumeral joint laxity under stress and non-stress conditions, they found that elite swimmers do not have excessive glenohumeral joint translation compared with age-matched nonswimming controls. Nor do they acquire excessive laxity as a result of their long-term participation in a sport with repetitive overhead activity.
Our results concur with the findings of Borsa et al48that repetitive swimming does not increase shoulder joint laxity in elite swimmers. These data also oppose the hypothesis that cumulative swimming time (weekly mileage×48 weeks×number of years coached) in the form of swimming training leads to glenohumeral joint laxity in swimmers. Hence, we doubt that laxity is a major contributor to swimmer’s shoulder.
On the other hand, our results showed a modest yet significant correlation between glenohumeral joint laxity and either impingement pain (rs=0.23, p<0.05) or extreme pain (rs=0.26, p<0.05). Similarly, McMaster et al14 found a statistically significant correlation between a clinical score of glenohumeral joint laxity and the presence of interfering shoulder pain in senior national and elite swimmers.
An alernate interpretation of swimmer’s shoulder
We found a high incidence of MRI-assessed symptomatic supraspinatus tendinopathy in elite swimmers. The incidence of tendinopathy was related to the time spent in training (hours swum per week) and the distance swum per week. Elite swimmers who trained for more than 15 h/week were twice as likely to have tendinopathy as those who trained for less time. Similarly, elite athletes who swam more than 35 km/week were four times more likely to have tendinopathy as those who swam fewer kilometres. The level of competition also correlated with supraspinatus tendinopathy, with a higher proportion of swimmers at the higher competition level having supraspinatus tendinopathy than swimmers at lower competitive levels.
An association between the extent of training and shoulder pain has been identified by Pollard49 when evaluating the prevalence of shoulder pain in elite British swimmers. Specifically, he found a correlation between the number of kilometres the athletes swam per week and shoulder pain (p<0.001). A study involving German triathletes also noted a significant relationship between the number of weekly training hours and incidence of muscle and tendon injuries (p<0.05).50Thus, shoulders of the elite swimmer are, in the long-term, susceptible to the continuous overloading associated with overuse activity.51 52 Our study indicates that supraspinatus tendinopathy in swimmers is related to the amount and duration of load on supraspinatus tendons. The high incidence of biceps tenderness and A-C joint pain suggest that these areas may also be subject to overload stress in elite swimmers.
Despite some evidence to the contrary by others,16 49 these data indicated that specific swimming strokes have little effect in predisposing elite swimmers to shoulder pain. In our view, the following diagram more accurately describes what generally happens to cause shoulder pain in elite swimmers.
In this model, repetitive movement causes tendinopathy with an associated increase in tendon thickness. Tendinopathy leads to pain when the thickened tendon and associated bursa are repeatedly squashed under the bony arch of the acromion during swimming as in impingement testing.
Our results showed that intensive training increases tendon thickness, a feature that is significantly associated with supraspinatus tendinopathy. Other human and animal studies have also shown that prolonged mechanical loading increases tendon thickness and alters its mechanical properties.21 53,–,57 For example, the cross-sectional area of the supraspinatus tendon was larger than normal in rats exercised for 4 weeks and continued to increase in size up to 16 weeks, its elasticity amounted to only 52–61% of the control (non-exercised) value, and less stress was required to rupture it. Following this overuse protocol, more substance of lesser quality was present in the injured tendons.21 In another study with exercised rats, the cross-sectional area of the Achilles tendon was increased at 4 months of running training.55 In horses, 18 months of training increased the cross-sectional area of the Achilles tendon, whereas 5 months of training did not.58 Also, a guinea fowl study showed that 8–12 weeks of mechanical loading was sufficient to increase tendon stiffness.56
A possible cellular mechanism for swimmer’s shoulder
Experimental studies suggest a mechanistic basis that may explain the supraspinatus tendinopathy we observed in the swimmers’ shoulders. For evaluating supraspinatus tendinopathy, the running rat model has been particularly informative.21 59 60 Supraspinatus tendon overuse was modelled by subjecting the rats to repetitive exercise, consisting of treadmill running at 17 m/min at a 10° decline for 1 h/day over 5 days/week.60 This protocol results in approximately 7500 strides/day.
In running rats, the supraspinatus tendon can be damaged by overuse and intrinsic factors, overuse and extrinsic factors, or overuse alone21 59 61 62. Specifically, extrinsic factors such as the coracoacromial arch can cause repetitive mechanical impingement of the rotator cuff. Intrinsic factors originate within the tendon as from tendon overload or tendon thickening. In combination, overuse plus extrinsic compression dramatically increased the incidence of significant injury in the rat supraspinatus tendon.62
Normally, the spindle-shaped tendon cells or tenocytes lie in rows surrounded by parallel bundles of collagen fibrils. Early microscopic change in rat supraspinatus tendons after running for 12 weeks is characterised by tenocyte rounding and proliferation. The cell machinery of the tenocytes is diverted from their differentiated task of maintaining the collagen fibrils and the extracellular matrix to mitosis. Under these conditions, the matrix accumulates water, glycosaminoglycans and other ground substance while collagen frays and becomes disorganised, and the tendon thickens. Loss of tendon biomechanical integrity occurs early, coinciding with tendon thickening. The structural changes are accompanied by decreased elasticity in the tendon and decreased load for its rupture.21
Mixed findings have been reported with regard to apoptosis and cellular inflammation in the tendons of rats that ran on a treadmill for 1 h/day over 1–3 months.63 64 Excessive apoptosis was induced in isolated rat tibialis anterior tendons loaded with a high (20%) strain for 6 h. Apoptotic rates were 20 times higher than in control tendons.65The biochemical basis for this apoptosis effect was examined using canine patellar tendon cells in tissue culture. The amount of stress applied directly to the tendon cells showed a positive relationship with the induction of a stress-activated protein kinase (c-Jun N-terminal kinase or JNK) within the cells.66 Cyclic strain (analogous to that in swimming movements) results in immediate activation of JNK, peaking at 30 min. This activation is regulated by a magnitude-dependent but not frequency-dependent, calciumdependent mechanotransduction pathway. Whereas transient JNK activation is associated with normal cell metabolism, persistent JNK activation can initiate apoptosis.66
In our study, the number of strokes per day calculated to be made by elite athletes who swam more than 15 h/week was similar to the number of strides per day made by the running rat model.60 62 At this activity level, significant change can occur in the thickness of the tendon and in supraspinatus tendinopathy. In the swimmers and the running rats, overuse activity can affect factors that are intrinsic (eg, tendon thickening) and extrinsic (eg, tendon impingement by the acromial arch). A weakness in our hypothesis is that we did not test whether scheduled rests can prevent or reverse supraspinatus tendinopathy in athletes. Soslowsky has shown this condition to be reversible in the running rat model (personal communication).
Although biopsies of the athletes’ tendons were not available to determine whether apoptosis occurs in human tendon cells stressed by overexercise, other studies in our laboratory based on discarded human tendon tissue obtained during surgery found that JNK is expressed, cells proliferate, collagen fibrils become disorganised, and apoptosis occurs in human rotator cuff tissue exposed to oxidative stress.67 68
In summary, these animal, ex vivo and in vitro findings help to explain our results in the elite athletes (figure 10). Both the swimming human and running rat systems showed significant associations between the amount of exercise (mileage/ time) and the development of tendinopathy, supporting the hypothesis that tendinopathy relates to the amount (mileage and hours) of load on the tenocytes that normally secrete procollagen and maintain the collagen fibrils. Under conditions of excessive stress, the homeostatic equilibrium may be lost, ultimately resulting in increased apoptosis, fraying and disorganisation of the collagen fibrils, water accumulation, tendon deterioration and pain. These changes could thus lead to the presence of incipient tears evident in some of the swimmers’ tendons.
Diagram showing how tendinopathies may arise.
Acknowledgments
The authors would like to thank the swimmers and their families, for participating in this study, and the South East Sydney and Illawara Area Health Service, for their support.
References
Footnotes
-
Funding This work was internally funded by the South East Sydney and Illawara Area Health Service.
-
Patient consent Obtained
-
Ethics approval The South East Sydney and Illawara Area Health Service