Article Text
Abstract
Background Methacholine hyperresponsiveness is prevalent in elite athletes. Comparative studies have hitherto been limited to methacholine, eucapnic voluntary hyperpnoea and exercise. This study investigated airway responsiveness to these stimuli as well as to adenosine 5′-monophosphate (AMP) and mannitol, in 58 cross-country ski athletes.
Methods Exhaled nitric oxide concentration (FENO), spirometry and bronchial challenge in random order with methacholine, AMP and mannitol were consecutively performed on three study days in the autumn. Specific IgE to eight aeroallergens and a self-completed questionnaire about respiratory symptoms, allergy and asthmatic medication were also performed on day 1. Eucapnic voluntary hyperventilation (EVH) and field exercise tests were randomly performed in 33 of the skiers on two study days in the following winter.
Results Of 25 (43%) skiers with airway hyperresponsiveness (AHR), 23, five and three skiers were hyperresponsive to methacholine, AMP and mannitol, respectively. Methacholine hyperresponsiveness was more prevalent in subjects without asthma-like symptoms. The FENO was not significantly different in skiers with and without methacholine hyperresponsiveness. Four of 14 skiers with and four of 19 skiers without methacholine hyperresponsiveness were hyperresponsive to EVH or exercise challenge. AHR to any stimulus was present in 16 asymptomatic and nine symptomatic skiers. Asthma-like symptoms were not correlated with AHR to any stimulus.
Conclusions Methacholine hyperresponsiveness is more common in asymptomatic skiers and is a poor predictor of hyperresponsiveness to mannitol and hyperpnoea. The low prevalence of hyperresponsiveness to indirect stimuli may suggest differences in the pathogenesis of methacholine hyperresponsiveness in elite skiers and non-athletes.
This paper is freely available online under the BMJ Journals unlocked scheme, see http://bjsm.bmj.com/info/unlocked.dtl
Statistics from Altmetric.com
Highly trained athletes commonly report respiratory symptoms, asthmatic medication use, asthma, airway hyperresponsiveness (AHR) and exercise-induced bronchoconstriction (EIB).1,–,7
AHR to indirect stimuli, such as exercise, eucapnic voluntary hyperventilation (EVH), hypo or hypertonic aerosols and adenosine 5′-monophosphate (AMP) is considered to be more specific for asthma than hyperresponsiveness to a direct stimulus such as methacholine. EVH is more sensitive than sport-specific field exercise or methacholine provocation and is the preferred test of the Medical Commission of the International Olympic Committee for the detection of EIB.8,–,10 Provocation with dry powder mannitol has recently been proposed as an alternative to EVH.11
Studies comparing airway responsiveness to different stimuli in elite athletes have hitherto been restricted to methacholine, EVH and field-based or laboratory exercise.9 12 13 In this study, we assessed AHR to methacholine, AMP and mannitol before and to EVH and a field exercise test during the competitive season and their association with self-reported respiratory symptoms, exhaled nitric oxide concentration (FENO) and allergic sensitisation in elite Norwegian cross-country skiers.
Methods
Subjects and study design
The study population consisted of 58 non-smoking cross-country and biathlon ski athletes (table 1). All subjects and parents of subjects under 18 years of age gave written informed consent. The study was approved by the Regional Ethics Committee in Trondheim.
Characteristics of study population
FENO, spirometry and bronchial provocation were consecutively performed on three study days in the autumn before the competitive season. The test sequence with methacholine, AMP and mannitol was determined by random allocation. A self-administered questionnaire on training hours, competitive experience, respiratory symptoms, allergy, use of asthmatic medication within the past year and asthma diagnosis and venepuncture were performed on day 1.
All subjects were invited 3–4 months later at approximately 1 month after the start of the competitive season for an EVH and a sport-specific field exercise test on two additional study days. Two subjects withdrew for personal reasons and 23 subjects did not complete the study, because of a current or recent upper respiratory tract infection or training commitments.
Bronchial provocation tests
Subjects were instructed to refrain from vigorous exercise within 4 h and using short-acting β2 agonists, cromones and ipratropium within 8 h, long-acting β2 agonists and antihistamines within 48 h and leucotriene receptor antagonists within 96 h of testing. Caffeine-containing drinks and inhaled corticosteroids were discouraged on the test day. Bronchial provocation was not performed within 6 weeks of an upper respiratory tract infection.
To control for diurnal variation in lung function, all tests were to be conducted at approximately the same time of day, defined as a maximal time difference of 90 min or less on any of two study days.
Lung function was assessed by spirometry (MasterScope spirometer; Erich Jaeger GmbH and Co KG, Hoechberg, Germany). The better of two measurements with less than 5% variation was recorded. Predicted normal values were based on reference values of Crapo et al.14
Doubling dose increments of methacholine 2.5 mg/ml (four increments) and 25 mg /ml (two increments) and AMP 25 mg/ml (four increments) and 250 mg/ml (three increments) were administered from the Spira Elektro 2 automatic inhalation synchronised dosimeter jet nebuliser (Respiratory Care Centre, Hameenlinna, Finland) by a controlled tidal volume breathing technique.7 15 The cumulative dose of methacholine and AMP were 1814 μg and 50.5 mg, respectively. Spirometry was performed 90 s after each increment and 180 s after the final increment. The test was terminated if the fall in the forced expiratory volume in 1 s (FEV1) was 20% or greater of the FEV1 measured after the inhalation of 0.9% NaCl.
A dry powder preparation of mannitol was delivered in gelatine capsules containing 0, 5, 10, 20 or 40 mg.16 Consecutive doses of 0, 5, 10, 20, 40, 80, 160, 160 and 160 mg to a cumulative dose of 635 mg were administered via an inhalator (Boehringer Ingelheim GmbH, Ingelheim, Germany) and a controlled deep inhalation to total lung capacity with 5 s of breathholding. Spirometry was performed 60 s after each dose. The test ended if the fall in FEV1 was 15% or greater of the FEV1 after inhalation of 0 mg mannitol or 635 mg had been inhaled.
For the EVH test, a dry gas mixture of 21% oxygen and 5% carbon dioxide in nitrogen (AGA AS, Oslo, Norway) at room temperature was administered through a mouthpiece attached to a bacterial filter, pneumotachograph, two-way non-rebreathing valve (T-Shape 2700; Hans Rudolph Inc, Shawnee, Kansas, USA) and two reservoir bags in series. Gas flow was regulated so that the bags were always fully inflated. With the help of a metronome subjects were encouraged to achieve a respiratory rate of 40 breaths/min and a minute ventilation of 30×baseline FEV1. The latter was measured at 30 s intervals. The average minute ventilation over the test duration of 8 min was calculated. Spirometry was performed before and at 3, 5, 7, 10 and 20 min after challenge.
The sport-specific field exercise test consisted of a ski run at competition intensity without a warm-up. Subjects were sent out in pairs whenever possible. The course was 4.7 km long and at a height of 118 m, increasing to a maximum height of 186 m over sea level at 1.8 km from the start. The ambient temperature varied from –13 to +2°C. Spirometry was performed before and at 5, 10, 15, 20 and 30 min after challenge.
Salbutamol 0.4 mg (Airomir Autohaler; 3M Pharma, UK) was administered if the FEV1 decline exceeded 15% after methacholine, AMP and mannitol and always at 20 and 30 min, respectively, after EVH and the sport-specific exercise tests. Spirometry was performed 10 min postadministration.
Allergic sensitisation
Serum was examined with the AlaTOP allergy screen (Immulite 2000; Diagnostic Products Corporation, Los Angeles, California, USA) for specific IgE to house dust mite, cat, dog, horse, timothy grass and birch pollens, mugwort and cladosporium. Sensitisation was defined as a specific IgE concentration of 0.7 IU/ml or greater.
Exhaled nitric oxide concentration
FENO was measured before challenge with methacholine, AMP and mannitol with the LR 2000 nitric oxide chemiluminescence analyser (Logan Research Ltd, Rochester, UK).17 Briefly, subjects exhaled from total lung capacity to residual volume at an expiratory flow rate of 250 ml/s and against a target resistance of 4–5 cm water with the help of a biofeedback monitor. FENO was the average of three measurements of the plateau of the exhaled nitric oxide curve.
Definitions
Asthma-like symptomatology was defined as wheeze and abnormal breathlessness or chest tightness, either on exertion, at rest or on exposure to irritants within the past year.
AHR to methacholine was defined as a provocative dose causing a 20% fall in FEV1 of 1814 μg or less, AMP as a provocative dose causing a 20% fall in FEV1 of 50.5 mg or less and mannitol as a provocative dose causing a 15% fall in FEV1 of 635 mg or less. AHR to EVH and sport-specific exercise challenge was defined as a fall in FEV1 over two time points of 10% or greater from baseline.
Ski asthma was defined as the presence of asthma symptomatology and AHR to methacholine.
Statistics
Statistical testing was performed with GraphPad Prism 4.02 for Windows. Differences in subject characteristics were analysed with the Student's t test for normally distributed data, Mann–Whitney U test for non-normally distributed data or the χ2 test (two-tailed p, Fisher's exact test when appropriate). Spirometry data were normally distributed and analysed by repeated measures analysis of variance and Tukey's multiple comparison test for all pairs of columns. FENO data were not normally distributed and were analysed with Friedman's test and Dunn's multiple comparison test. Correlation coefficients were calculated using Spearman's rank method. Statistical significance was defined as a p value of 0.05 or less.
Results
All tests were performed without complications. For methacholine, AMP and mannitol tests the mean (range) interval was 29.5 (6–50) days. The mean maximal time difference for testing was 106 (0–405) min on any of two study days and within 90 min in 38 (66%) subjects. For EVH and field exercise tests, the interval between tests was 9.2 (2–20) days, the mean maximal time difference was 149 (0–340) min and was within 90 min in 13 (39%) subjects.
AHR to methacholine, AMP and mannitol
Twenty-five (43%) subjects were hyperresponsive to one of these stimuli. Hyperresponsiveness to methacholine was present in 23 subjects (median (interquartile range; IQR) PD20 FEV1 486 μg (342–929)) and to AMP in five (8.3%) subjects (median (IQR) PD20 FEV1 35.5 mg (15.8–37.4)). Three (5.1%) subjects were hyperresponsive to mannitol, with individual PD15 FEV1 of 315, 560 and 635 mg.
The response–dose ratios for AMP and mannitol were not significantly different in skiers with and without AHR to methacholine (figure 1). There was a significant correlation between the decrements in FEV1 during AMP and mannitol provocation (N=58, rs=0.33, p=0.011).
Response–dose ratios for adenosine 5′-monophosphate (AMP) (A) and mannitol (B) in 58 skiers by hyperresponsiveness to methacholine. Response–dose ratio at or below stippled line indicates hyperresponsiveness to AMP and mannitol. Bars represent median values. FEV1, forced expiratory volume in 1 s.
Baseline FEV1 (mean (SD) L, % predicted (SD)) on each study day was within normal limits (AMP 4.04 (0.74), 103.3 (9.6); mannitol 4.02 (0.77)*, 102.5 (9.7); methacholine 4.12 (0.79), 105.1 (10.3)). FEV1 was significantly lower before challenge with mannitol than with methacholine (*p<0.05). Forced vital capacity and FEV1/forced vital capacity ratios were also within normal limits (data not shown).
AHR to EVH and field exercise
Average minute volume (mean (SD)) during EVH was 108.2 (24.7) L and 77.6 (10.2) % of maximum minute ventilation.
Of 33 skiers, three (9%) and six (18%) skiers were hyperresponsive to EVH and field exercise tests, respectively. In those without previous methacholine hyperresponsiveness, AHR to either stimulus was detected in four subjects. No subject was positive to both tests. Of 14 (42%) skiers with methacholine hyperresponsiveness (median (IQR) PD20 FEV1 methacholine 396.5 μg (303.5–904.5)), three were hyperresponsive to either test and one was hyperresponsive to both tests.
The area under the curve (AUC) (mean (SD) % fall FEV1 min) after EVH was not significantly different in skiers with and without methacholine hyperresponsiveness (AUC0–20 –53 (56) vs –6 (59), p=0.061) or field exercise test (AUC0–30 –110 (152) vs –19 (131), p=0.14). AHR to any of the five stimuli was present in 20 (61%) skiers (figure 2).
Interrelationship of airway hyperresponsiveness to methacholine, adenosine 5′-monophosphate (AMP), mannitol and hyperpnoea (eucapnic voluntary hyperventilation and field exercise test) in 33 skiers.
AHR, asthma symptomatology and doctor-diagnosed asthma
The relationship between asthma symptomatology and AHR to methacholine, AMP and mannitol is summarised in figure 3. Asthma-like symptomatology was not associated with AHR to any stimulus, with AHR to methacholine being present in seven (27%) and 16 (50%) skiers with and without symptoms, respectively. The geometric mean PD20 FEV1 for symptomatic and asymptomatic skiers was 360.0 μg (CI 167.2 to 775.3) and 606.1 μg (CI 448.7 to 818.8), p=0.17, respectively.
Prevalence of airway hyperresponsiveness to methacholine, adenosine 5′-monophosphate (AMP) and mannitol in 58 skiers related to self-reported asthma symptomatology (wheeze and abnormal breathlessness or chest tightness, either on exertion, at rest or on exposure to irritants within the past year).
In the methacholine-positive skiers, four reported doctor-diagnosed asthma. Of these, two were on inhaled corticosteroids and were also hyperresponsive to AMP. One of these skiers was also hyperresponsive to mannitol.
In the methacholine-negative skiers, doctor-diagnosed asthma was reported by six subjects. Of these, three reported the use of inhaled corticosteroids, one was hyperresponsive to mannitol and another was hyperresponsive to AMP.
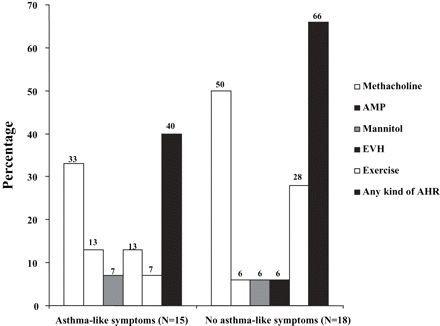
The distribution of AHR to methacholine, AMP, mannitol, EVH and field exercise by asthma-like symptomatology for 33 skiers is presented in figure 4. One skier with AHR to EVH and five skiers with AHR to exercise did not previously report asthma-like symptoms. One skier with AHR to both tests reported asthma-like symptoms.
Prevalence of airway hyperresponsiveness (AHR) to methacholine, adenosine 5′-monophosphate (AMP), mannitol eucapnic voluntary hyperventilation (EVH) and field exercise related to asthma symptomatology in 33 skiers.
Exhaled nitric oxide concentration
FENO was measured in 44 subjects before challenge with mannitol, AMP and methacholine. The median (IQR) FENO on mannitol, methacholine and AMP challenge days was 4.1 ppb (3.5–5.4), 6.7 ppb (4.9–8.4) and 5.2 ppb (3.9–7.6) (p<0.001), respectively. FENO was significantly higher on the methacholine (p<0.001) and AMP (p<0.01) challenge days than on the mannitol challenge day.
FENO before methacholine challenge was not significantly different in skiers with and without hyperresponsiveness to methacholine (median (IQR) 7.3 (4.3–8.6) vs 6.5 (5.2–8.2) p=0.89). There was no correlation between FENO and the response–dose ratio for methacholine (N=58, rs=−0.074, p=0.848).
Allergic sensitisation
Allergic sensitisation was present in 12 of 16 subjects with self-reported allergy, five of 33 subjects without self-reported allergy and in three of nine subjects who were uncertain about their allergy status.
Discussion
The airways of elite skiers clearly react in a heterogeneous manner in the training season in the autumn, being more responsive to methacholine than to AMP and mannitol. Methacholine hyperresponsiveness, defined as a PD20 FEV1 of 1800 μg or less, was present in 40% (23) of skiers. By contrast, provocation with AMP and mannitol identified AHR only in five and three subjects, respectively. Additional provocation with EVH and field exercise tests at 1 month into the competitive season detected hyperresponsiveness in eight skiers.
A number of studies report a high prevalence of AHR in winter athletes.4 7 18 19 This study confirms that high prevalence and extends the finding to report that the AHR to methacholine was more prevalent in those not reporting asthma-like symptoms. Furthermore, asthmatic airway inflammation was not a prerequisite for AHR to methacholine. The values for FENO were normal and consistent with the mild AHR with a PD20 of 486 µg (342–929). In the skiers with methacholine hyperresponsiveness, 10 (17%), eight steroid-naive skiers with a PD20 FEV1 of 400 μg or less and two skiers on inhaled corticosteroids would have satisfied the criteria for a therapeutic use exemption for β2 agonists for the 2008 Olympic Games.20 An additional four skiers would have qualified by way of the hyperpnoea stimulus.
The reason for the high prevalence of AHR to methacholine in winter athletes is unclear. One possibility is that breathing large volumes of unconditioned air over long periods during training may cause injury to the airway epithelium,21 22 and simply enhance access of the methacholine to the M3 muscarinic receptor on bronchial smooth muscle. The plasma exudation that follows repeated injury may lead to bronchial smooth muscle itself becoming more responsive through a change in its contractile properties.21 Another possibility is that the increased cholinergic tone in elite athletes23 may increase the receptor sensitivity. Finally, there may have been underreporting and/or reduced perception of symptoms.
In contrast, in the summer athletes reported by Holzer et al10 and Pedersen et al13 there was a lower prevalence of methacholine AHR and a higher prevalence of AHR to indirect stimuli such as EVH and mannitol11 13 The athletes studied by Holzer and colleagues,10 11 however, did have a higher prevalence of asthma diagnosis relative to those in the current study. Furthermore, there is a greater frequency of allergic sensitisation in summer athletes.24 25 In addition, there is a reduced likelihood of injury to the airways from the need to condition air when exercise is performed under warmer and more humid conditions of summer or in swimming pools.
The indirect stimuli used here are thought to induce bronchoconstriction by the release of mast cell mediators.26,–,29 Four skiers had classic allergic asthma, with AHR to either AMP or mannitol or both. The low prevalence of hyperresponsiveness to AMP in the present study corroborates our previous findings in another population of cross-country skiers.15 The prevalence of mannitol hyperresponsiveness in our study is in accordance with that of 9.2%, recently reported by Lund et al30 in Danish elite athletes.
Which stimulus should be employed to assess AHR in skiers with respiratory symptoms? In the present study, methacholine hyperresponsiveness that would satisfy International Olympic Committee criteria was present in only 10 of the skiers. For skiers with a negative methacholine test, mannitol provocation detected hyperresponsiveness in only one other skier, and EVH and field exercise during the competitive season identified hyperresponsiveness in two other skiers. In contrast, EVH was superior to methacholine in both elite Danish swimmers and Australian summer sports athletes for diagnosing and evaluating EIB.10 13 Stensrud et al18 have demonstrated that hyperpnoea during field exercise is less sensitive than methacholine for identifying hyperresponsiveness in elite skiers. Increased airway responsiveness to mannitol has a low prevalence in Scandinavian athletes compared with the Australian athletes studied by Holzer et al.11 Paradoxically, methacholine responsiveness is greater in athletes from Scandinavia. The reasons for these differences are unknown, but may relate to differences in allergic sensitisation or between the quantity and condition of air during training between the two regions during several seasons. Although studies do not give any clear indication with regard to the preferred stimulus, it would be intuitive to use hyperpnoea as the stimulus, as symptoms in athletes are exercise related. If this stimulus had only been used then eight of 33 (24%) would have been identified to satisfy the International Olympic Committee criteria.
Our study was limited by the EVH and field exercise tests being performed several months after the other tests and in only 57% of the original group. Although baseline FEV1 values were not significantly different on each test day (data not shown), selection bias may have influenced the prevalence of hyperresponsiveness to EVH and field exercise. However, tests were performed in 24 of 26 skiers who reported asthma-like symptomatology at inclusion. In addition, responsiveness to methacholine, AMP or mannitol may have altered in the intervening period. Indeed, an increase in methacholine responsiveness by more than twofold has been reported during the competitive part of the season.31 32 However, the training burden is reduced during this period and a decrease in methacholine responsiveness cannot be excluded. Irrespective of the shift in responsiveness, the lower prevalence of AHR to EVH and a field exercise test cannot be attributed to an inadequate stimulus. The EVH test was over 8 min instead of the conventional 6 min, and the 4.7 km ski run was at competition intensity and without a warm-up. We used two time points with a 10% or greater fall for a positive test, whereas others may have used only one.12 With one, the number would rise to 14.
In conclusion, airway responsiveness to direct and indirect bronchoconstrictive stimuli is heterogeneous in elite cross-country skiers. Methacholine hyperresponsiveness is more prevalent in skiers without asthma-like symptoms than in skiers with asthma-like symptoms. The extremely low prevalence of hyperresponsiveness to the indirect stimuli of AMP, mannitol, EVH and field exercise suggest a different pathogenesis for methacholine hyperresponsiveness in elite skiers and non-athletes. This may be related to allergic sensitisation or training environment with possible consequences for the diagnosis and management of symptoms and AHR in these athletes.
What is already known on this topic
▶. AHR to methacholine in winter athletes is commonly used as objective evidence to justify the use of asthma medication.
What this study adds
▶. This study demonstrates that AHR to methacholine is more prevalent in asymptomatic than in symptomatic skiers.
▶. Airway injury during training may be important in the pathogenesis of AHR to methacholine in winter athletes.
References
Footnotes
-
Funding MS-C was financially supported as a post-doctoral research fellow by the Norwegian Research Council. The study was also supported by a study grant from Glaxo SmithKline, Norway.
-
Competing interests None for MS-C, NC and LB. SDA is the inventor of the mannitol test. The intellectual property is owned by Sydney South West Area Health Service (SSWAHS) and the commercial rights are licensed to Pharmaxis Ltd (Frenchs Forest, NSW, Australia). SDA and JDB own shares in Pharmaxis Ltd, which they purchased themselves but they have not received options. SDA and JDB each receive a 10% share of the royalties paid to SSWAHS.
-
Ethics approval This study was conducted with the approval of the Regional Ethics Committee in Trondheim.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.