Article Text
Abstract
Aim The Royal Dutch Society for Physical Therapy (KNGF) instructed a multidisciplinary group of Dutch anterior cruciate ligament (ACL) experts to develop an evidence statement for rehabilitation after ACL reconstruction.
Design Clinical practice guideline underpinned by systematic review and expert consensus.
Data sources A multidisciplinary working group and steering group systematically reviewed the literature and wrote the guideline. MEDLINE and the Cochrane Library were searched for meta-analyses, systematic reviews, randomised controlled trials and prospective cohort studies published between January 1990 and June 2015.
Eligibility criteria for selecting studies Included literature must have addressed 1 of 9 predetermined clinical topics: (1) preoperative predictors for postoperative outcome, (2) effectiveness of physical therapy, (3) open and closed kinetic chain quadriceps exercises, (4) strength and neuromuscular training, (5) electrostimulation and electromyographic feedback, (6) cryotherapy, (7) measurements of functional performance, (8) return to play and (9) risk for reinjury.
Summary Ninety studies were included as the basis for the evidence statement. Rehabilitation after ACL injury should include a prehabilitation phase and 3 criterion-based postoperative phases: (1) impairment-based, (2) sport-specific training and (3) return to play. A battery of strength and hop tests, quality of movement and psychological tests should be used to guide progression from one rehabilitation stage to the next. Postoperative rehabilitation should continue for 9–12 months. To assess readiness to return to play and the risk for reinjury, a test battery, including strength tests, hop tests and measurement of movement quality, should be used.
- Anterior cruciate ligament
- Rehabilitation
Statistics from Altmetric.com
Introduction
Anterior cruciate ligament reconstruction (ACLR) is a common treatment for athletes after ACL injury. The incidence of non-contact ACL injuries appears to be the greatest in athletes who are between 15 and 40 years of age and participate in pivoting sports like soccer, handball, volleyball and alpine skiing.1 ,2 Every year, about 3% of amateur athletes injure their ACL; for elite athletes, this percentage could be as high as 15%.2 Females are two to eight times more likely to sustain an ACL injury than their male counterparts, probably because male and female neuromuscular patterns diverge during and following puberty.3–8
Besides its mechanical function in maintaining knee stability, the ACL contains mechanoreceptors (2.5%) and therefore directly influences the neuromuscular control of the knee.9 ACL deficiency causes partial deafferentiation and alters spinal and supraspinal motor control. The changes in motor control strategy can reveal changes in proprioception, postural control, muscle strength, movement and recruitment patterns.10 An ACL injury might therefore be regarded as a neurophysiological dysfunction and not a simple peripheral musculoskeletal injury.11 ,12 It is also not self-evident that an ACLR will automatically lead to a return to preinjury activity level.
Recent research shows that 35% of athletes after ACLR do not return to preinjury sport level within 2 years.13–15 Half of these athletes report their ACL injury as the primary reason for a lower activity level.13 ,14 ,16–18 Apart from the physical recovery, also the psychological response (eg, fear of reinjury) after ACLR has an influence on whether an athlete chooses to return to play.19–25 Return to play is defined as the ability to play a competitive match at the preinjury level. Moreover, recent research shows that 3–22% of athletes rerupture the reconstructed ligament and 3–24% rupture the contralateral ACL in the first 5 years after ACLR.17 ,26–30
The difficulty with determining the moment of return to play is that it is unknown which measures should be used to predict a safe return to play with a low risk of a second ACL injury. Three recent systematic reviews show that the return-to-play decision by clinicians is hardly based on objective clinimetric criteria.27 ,31 ,32 Furthermore, these studies concluded that return to play is only connected to quantitative criteria, while it is known that qualitative criteria (eg, dynamic knee valgus, knee flexion angle and trunk control) play an important role in prevention and rehabilitation. Movement quality actually may affect the ACL (re)injury rate.33 ,34 The occurrence of dynamic knee valgus when landing from a jump, for instance, increases the risk of ACL (re)injury.35 ,36
Return to play is the ultimate goal of rehabilitation programmes. So the above-mentioned factors are important topics to incorporate in the rehabilitation process after ACLR. However, currently, there is no consensus regarding the content of a rehabilitation programme. Therefore, the Royal Dutch Society for Physical Therapy (KNGF) instructed a multidisciplinary group of ACL experts in the Netherlands to develop an evidence statement for anterior cruciate ligament rehabilitation. The goal of this evidence statement was to describe the rehabilitation after ACLR and to encourage uniformity in physical therapy treatment and use of measurements of functional performance. The following three questions were formulated by a steering group of the KNGF to guide the realisation of the evidence statement:
What should be the content of the rehabilitation protocol after ACLR based on scientific evidence and, if not present, based on best practice?
Which measurements and assessments can be applied to monitor progression during the rehabilitation programme and to determine outcomes at the end of rehabilitation programme?
What criteria should be used to determine the moment of return to play?
Methods
Expert participants
The process started with the formation of a multidisciplinary working group and steering group. The working group consisted of six Dutch ACL experts with 8–35 years of experience in ACL rehabilitation: five physical therapists specialised in sports injury rehabilitation and one orthopaedic surgeon specialised in knee surgery, ACL surgery in particular. The steering group consisted of ACL experts from different professions with 10–37 years of experience in ACL rehabilitation (three physical therapists, one sports physician, one orthopaedic surgeon and one trauma surgeon).
Procedure
The first author (NvM) chaired the working group and was responsible for the systematic review steps (literature search, methodological quality assessment, data extraction, data analysis, description of the results and translation into practice guidelines) and for writing the evidence statement. The working group monitored each step in the systematic review process and assisted in methodological quality assessment of the included studies, the writing process and the translation into practice guidelines. The steering group (chairman REHvC) validated all steps made by the first author and the working group. The KNGF assisted in the administrative processes.
The working group contacted each other by email and every 2 months a consensus meeting was organised. Every other meeting, the steering group joined the working group.
The first meeting of the working and steering group together, started with the formulation of nine clinical topics important for ACLR rehabilitation. These topics were used to guide the systematic review process. These nine topics were: (1) preoperative predictors for postoperative outcome, (2) effectiveness of physical therapy, (3) open kinetic chain (OKC) versus closed kinetic chain (CKC) quadriceps exercises, (4) strength training and neuromuscular training, (5) electrostimulation and electromyographic feedback, (6) cryotherapy, (7) measurements of functional performance, (8) return to play and (9) risk of reinjuries.
Articles found during the systematic review process were subdivided into the nine topics and every topic was given a level of evidence according to the EBRO (Dutch evidence-based guideline development) criteria.37 The recommendations were, if available, based on the latest scientific evidence, supplemented with best practice when necessary. The results of the systematic review process (see online supplementary appendix 1) were used to formulate the evidence statement (see online supplementary appendix 2).
supplementary appendix
supplementary appendix
Search strategy
A systematic literature search was performed searching in MEDLINE (PubMed) and the Cochrane Library to identify relevant articles from January 1990 up to June 2015 using keywords specified for the database according to the nine topics mentioned above with PICO questions (table 1). An academic librarian composed a syntax based on all the keywords. Meta-analyses, systematic reviews, randomised controlled trials (RCTs) and prospective cohort studies were included for study selection.
Search strategy 31 May 2015
Study selection
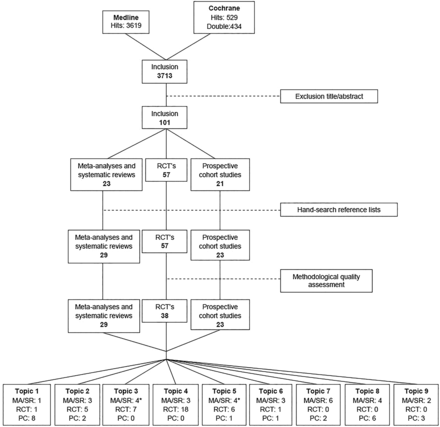
All eligible articles were screened first by title and abstract independently by two reviewers (NvM and REHvC). When the two reviewers did not reach consensus, a third reviewer (CN) made the final decision. After this first inclusion, the full-text article was screened using the inclusion and exclusion criteria as listed in table 2. In addition, a hand search was performed on the reference lists of meta-analysis and systematic reviews for RCTs and prospective cohort studies that were not included in the primary search. A flow chart of the search strategy is presented in figure 1.
Inclusion and exclusion criteria for literature search
Flow chart of search strategy on 31 May 2015. *Topics 3 and 5 share one SR. MA, meta-analysis; PC, prospective cohort study; RCT, randomised controlled trial; SR, systematic review.
Methodological quality assessment
Quality assessment of the included articles was independently performed by two reviewers (NvM and REHvC). When the reviewers did not reach consensus, a third reviewer made the final decision. All articles were individually graded for level of methodological quality (table 3 and online supplementary appendix 1).
Grading of the level of methodological quality of individual studies (EBRO)
Methodological quality of meta-analyses and systematic reviews was assessed with the AMSTAR checklist. The assessment of risk of bias of the RCTs was performed with the PEDro scale (http://www.pedro.org.au). The PEDro scale was scored on 10 items. Methodological quality was rated poor when an article had a score of ≤4. Subsequently, the RCTs with poor quality were excluded.
The prospective cohort studies were assessed with an adapted Cochrane Library Checklist (table 4), also used before in the KNGF guideline for urinary incontinence.38 This checklist has a maximum score of 5. Prospective cohort studies were only used when no higher level evidence was available or to support findings in the RCTs.
Adapted Cochrane library checklist
Data extraction
Data extraction was performed by one reviewer (NvM). See online supplementary appendix 1 for the data extraction table. Results from the included studies were synthesised descriptively for the evidence statement. Based on the results of all articles selected in one topic, a final conclusion was made with a corresponding level of evidence (table 5).37 ,39 To correct for double evidence, RCTs that were also included in a meta-analysis or systematic review were not used separately to determine the level of evidence of the final conclusion.
Level of evidence of the conclusion (EBRO)
Results
Study selection and methodological quality assessment
After removing doubles, the systematic literature search in MEDLINE and the Cochrane Library provided 3713 articles (figure 1). After the first exclusion based on title and abstract, 101 articles were included for full-text assessment. After reading, no study was excluded. After the hand search in the reference lists of meta-analyses and systematic reviews, eight articles were included additionally. After quality assessment, 19 RCTs were excluded based on a PEDro score of ≤4. The most common flaws were no blinding of participants, therapists or outcome assessors and an inadequate percentage of participants eligible for follow-up.
All final included (n=90) articles were arranged by topic: 10 for preoperative predictors for postoperative outcome,40–49 10 for effectiveness of physical therapy,50–59 11 for OKC versus CKC quadriceps exercises,60–70 21 for strength training and neuromuscular training,71–91 11 for electrostimulation and electromyographic feedback,63 ,93–102 5 for cryotherapy,103–107 8 for measurements of functional performance,27 ,31 ,32 ,108–112 10 for return to play13 ,14 ,17 ,22 ,113–118 and 5 for risk of reinjuries29 ,35 ,36 ,119 ,120 (topics ‘open vs closed kinetic chain quadriceps exercises’ and ‘electrostimulation’ share one systematic review).
Data extraction
Evidence for clinical practice at all nine topics is summarised below, according to table 5. See also online supplementary appendix 1 for the data extraction table. Final recommendations were made according to the EBRO criteria in table 5.
Preoperative predictors for postoperative outcome
Ten articles were found about preoperative predictors for postoperative outcome. These were one systematic review,40 one RCT41 and eight prospective cohort studies.42–49
The prospective cohort studies of Eitzen et al,42 Heijne et al,44 McHugh et al47 and McHugh et al48 were included in the systematic review of de Valk et al.40 This level A2 systematic review documented that (1) better functional outcomes after ACLR were achieved for men than for women at a minimum follow-up of 1 year after ACLR, no matter the graft choice; (2) patients younger than 30 years of age had a higher postoperative Tegner activity level than older patients at a minimal follow-up of 22 months after ACLR; (3) patients with ACLR within 3 months after injury and patients with a high preoperative Tegner activity level have a higher Tegner activity level at a minimal follow-up of 2 years after ACLR; (4) smoking, high BMI (>30), quadriceps strength deficits and range of motion (ROM) deficits resulted in worse functional outcomes at a minimum of 1 year after ACLR.40 The steering group found some prospective cohort studies that supported the conclusions of de Valk et al. Lepley and Palmieri-Smith45 (level B) showed that preoperative quadriceps strength is positively related to postoperative quadriceps strength at the moment of return to play. Månsson et al46 (level C) found that a higher preoperative Tegner activity level predicts a better outcome at a minimal follow-up of 22 months. Quelard et al49 (level B) described that a limited preoperative ROM and female sex account for a limited ROM 3 months postoperative.
Grindem et al43 (level C prospective cohort study) and Shaarani et al41 (level B RCT) investigated the effect of preoperative rehabilitation, so-called prehabilitation, on the outcome after ACLR. Grindem et al43 described that combined prehabilitation and postoperative rehabilitation had better self-reported knee function at 2-year follow-up compared to postoperative rehabilitation only. Shaarani et al41 had a follow-up of only 12 weeks after ACLR. They found no differences in quadriceps and hamstring (HS) strength between a prehabilitation group and a group with no prehabilitation, but the prehabiliation group scored better on self-reported knee function.41
From the above-mentioned predictive factors, the non-modifiable factors could be taken into account by the physical therapist to predict the outcome of treatment.
The conclusions about modifiable factors in this topic were as follows:
Level 2: a preoperative extension deficit (lack of full extension) is a major risk factor for an extension deficit after ACLR.40 ,49
Level 2: a preoperative deficit in quadriceps strength of >20% has a significant negative consequence for the self-reported outcome 2 years after ACLR.40 ,45
Level 3: prehabilitation ensures better self-reported knee function up to 2 years after ACLR.41 ,43
Effectiveness of physical therapy
Ten articles were found about the effectiveness of postoperative physical therapy. These were three systematic reviews,50–52 five RCTs53–57 and two prospective cohort studies.58 ,59
The systematic review of van Grinsven et al51 (level B) described a time-based rehabilitation protocol based on the available evidence supplemented with expert opinion.
The level A1 systematic review of Coppola and Collins50 investigated the effect of physical therapy after knee surgery. Based on 10 RCTs, they concluded that physical therapy is not more effective than a home exercise programme in a young and healthy population following relatively simple knee surgery as arthroscopic meniscectomy. However, for rehabilitation after complicated knee surgery as ACLR, there is a lack of evidence.50 The systematic review of Wright et al52 (level A1) concluded that it is reasonable that a minimally supervised rehabilitation can result in successful ACLR rehabilitation. In their study, Coppola and Collins50 included three RCTs about rehabilitation after ACLR. Wright et al52 included the same three RCTs plus the RCT of Beard and Dodd.53 The level B RCT of Beard and Dodd53 showed that physical therapy had minimal extra benefit in a, not explicitly described, young athletic population after ACLR. Their rehabilitation programme was only administered from weeks 4 to 16 after ACLR. They found no differences in self-reported knee function and quadriceps and HS strength 24 weeks after ACLR.53 Hohmann et al57 and Grant and Mohtadi56 (both level B) also investigated the difference between supervised physical therapy (Hohmann: 19 sessions, Grant and Mohtadi: 17 sessions) versus home-based rehabilitation (4 sessions). They both found no between-group differences in ROM, quadriceps and HS strength and hop tests at >1 year follow-up,56 ,57 but Grant and Mohtadi56 found a better self-reported knee function in the home-based group. The level C prospective cohort study of Dragicevic-Cvjetkovic et al58 found a better self-reported knee function and greater improvement in thigh muscle circumference in a rehabilitation group (20 weeks) compared to a group with no rehabilitation at all at a 1 year follow-up.
Both studies of Beynnon et al54 ,55 (levels B and A2) studied the difference between a 19-week and a 32-week rehabilitation programme after ACLR. They concluded that there were no differences in self-reported knee function, laxity, ROM, strength and hop tests at a 2-year follow-up.54 ,55 The rehabilitation programme of Muneta et al59 (level B) comprised a 6-month rehabilitation. Their results are comparable to both studies of Beynnon et al.54 ,55 ,59
The conclusions in this topic were as follows:
Level 2: owing to a lack of high-quality studies and contradictory results, it is unclear whether there is a benefit of supervised rehabilitation compared to home-based rehabilitation or no rehabilitation at all. A minimally supervised rehabilitation programme may result in successful rehabilitation in specific groups of patients that are highly motivated and live far from a physical therapist.50 ,52 ,56 ,57
Level 2: when comparing a 19-week with a 32-week rehabilitation programme, there are no differences in terms of laxity, ROM, self-reported knee function, single-leg hop test for distance or isokinetic concentric quadriceps and HS strength.54 ,55 ,59
OKC versus CKC quadriceps exercises
Concerning the OKC and CKC quadriceps exercises, 11 articles were traced. These were four systematic reviews60–63 and seven RCTs.64–70
Andersson et al60 conclude in their systematic review (level A1) that after ACLR with BTPB, CKC quadriceps exercises produce less pain, less risk of increased laxity and better self-reported knee function compared to OKC quadriceps exercises. They included the RCTs of Bynum et al,64 Mikkelsen et al,67 Morrissey et al68 and Perry et al.69 The recent RCT of Uçar et al70 found no differences between CKC and OKC exercises, but they investigated a group of patients after ACLR with a HS graft.
The systematic reviews of Glass et al61 (level A1) and Wright et al63 (level A1) conclude that OKC quadriceps exercises should not be used in the first 6 weeks of rehabilitation after ACLR. Herewith, they confirmed the results of Andersson et al.60 The RCT of Heijne and Werner66 (level B) investigated early (4 weeks) versus late (12 weeks) start of OKC quadriceps exercises and compared ACLR with bone-patellar tendon-bone (BPTB) and HS. They concluded that the HS group with an early start had more laxity after a follow-up period of 7 months than the other groups. Besides, an early start of OKC quadriceps exercises had no beneficial effect on quadriceps strength.66 Fukuda et al65 (level B RCT) described that OKC quadriceps exercises can be started from week 4 after ACLR with HS, but in a limited ROM between 45° and 90°.
The systematic review of Lobb et al62 concluded that there is limited evidence that a combination of OKC and CKC quadriceps exercises results in better strength and return to play than CKC exercises alone. They also included the systematic review of Andersson et al.60 ,61
The overall conclusions were as follows:
Strength training and neuromuscular training
Concerning strength training and/or neuromuscular training, 21 articles were found. Among them were 3 systematic reviews71–73 and 18 RCTs.74–91
Systematic reviews of Gokeler et al72 (level A1) and Kruse et al73 (level A1) concluded that eccentric quadriceps training can be safely incorporated 3 weeks after ACLR and may be the most effective way of restoring quadriceps strength. However, the level A1 systematic review of Augustsson71 concluded that the strength training programmes after ACLR should be further developed because it is still unclear what is the best way to train the quadriceps. To optimise outcome after rehabilitation, neuromuscular training should be added to strength training according to Gokeler et al72 and Kruse et al.73 Neuromuscular training is defined as training enhancing unconscious motor responses by stimulating afferent signals and central mechanisms responsible for dynamic joint control.92 These exercises are designed to induce compensatory changes in muscle activation patterns and facilitate dynamic joint stability.92 Nine RCTs were included in the above-mentioned systematic reviews: Cooper et al,78 Gerber et al,80 Gerber et al,82 Risberg et al,87 Risberg and Holm,88 Sekir et al89 and Shaw et al.90 The level B RCTs of Berschin et al,75 Bieler et al,76 Fu et al,79 Gerber et al81 and Kinikli et al84 support the findings in those systematic reviews.
The level B RCTs of Isberg et al83 and Shaw et al90 concluded that isometric quadriceps exercises are safe in the first postoperative weeks, because there are no differences in laxity up to 2 years of follow-up.
Baltaci et al74 (level B RCT) and Cappellino et al77 (level B RCT) demonstrated that the use of Wii Fit, respectively, neurocognitive rehabilitation have no beneficial effect to a combined strength and neuromuscular rehabilitation at a short-term follow-up.
Tyler et al91 (level B RCT) concluded that immediate weight bearing had no detrimental effects for laxity and a positive effect on anterior knee pain at a 1-year follow-up.
The main conclusions were as follows:
Level 1: starting eccentric quadriceps training (in CKC) from 3 weeks after ACLR is safe and contributes to a bigger improvement in quadriceps strength than concentric training.72 ,73 ,76 ,81 ,84
Level 1: neuromuscular training should be added to strength training to optimise self-reported outcome measurements.72 ,73 ,75 ,79
Level 2: isometric quadriceps exercises are safe from the first postoperative week.83 ,90
Level 3: immediate weight bearing does not affect knee laxity and results in decreased incidence of anterior knee pain.91
Electrostimulation and electromyographic feedback
Eleven articles about electrostimulation and electromyographic feedback were found. These were four systematic reviews,63 ,93–95 six RCTs96–101 and one prospective cohort study.102
Imoto et al93 and Kim et al94 (level A1 systematic reviews) concluded that the addition of electrostimulation to conventional rehabilitation might be more effective in improving quadriceps strength up to 2 months after ACLR. The level A2 RCT of Paternostro-Sluga et al100 and the level B RCT of Fitzgerald et al99 were included in both systematic reviews. Ediz et al97 (level B RCT) and Lepley et al102 (level C prospective cohort study) found no differences in effusion, pain, ROM and knee extension and flexion moments when electrostimulation was added to conventional rehabilitation. Feil et al98 and Taradaj et al101 (both level B RCTs) did examine quadriceps strength and found a higher increase in quadriceps strength when electrostimulation was added to conventional rehabilitation at a 6-month follow-up. Wright et al63 (level A1 systematic review) summarised that electrostimulation may help improve quadriceps strength in the early postoperative period, but that it is not a prerequisite for successful rehabilitation. All authors did not distinguish between regaining quadriceps motor control and increasing quadriceps strength.
Studies concerning electromyographic feedback are contradictory. The systematic review of Wasielewski et al95 (level A1) showed that electromyographic feedback improves short-term postsurgical pain after ACLR, but Christanell et al96 (level B RCT) described no differences in pain during the first six postoperative weeks with or without biofeedback.
The conclusions on this topic were as follows:
Level 1: electrostimulation, in combination with conventional rehabilitation, might be more effective for improving muscle strength for up to 2 months after ACLR than conventional rehabilitation alone. However, its effect on long-term functional performance and self-reported knee function is inconclusive.63 ,93 ,94 ,98 ,101
Level 2: electromyographic feedback might improve short-term postsurgical pain after ACLR.95 ,96
Cryotherapy
Five articles were found about cryotherapy: two meta-analyses,103 ,104 one systematic review,105 one RCT106 and one prospective cohort study.107
All three level A1 articles shared the conclusion that cryotherapy is effective in reducing postoperative pain until about 1 week postsurgery, but it has no effect on drainage or ROM.103–105 The level A2 RCT of Edwards et al106 was included in the meta-analyses of Martimbianco et al.103 The prospective cohort study of Glenn et al107 (level C) supports these findings.
The conclusion on this topic was as follows:
Measurements of functional performance
Eight articles about measurements of functional performance were traced: six systematic reviews23 ,27 ,28 ,108–110 and two prospective cohort studies.111 ,112
Five systematic reviews (all level B) concluded that there is a lack of objective criteria to determine return to play.23 ,27 ,28 ,109 ,110 Extensive test batteries for determining quantity and quality of movement are recommended, including strength tests, hop tests and video analysis for measuring quality of movement.27 ,28
There is weak evidence from a level A2 systematic review for factors that could be associated with a higher chance of return to play: less effusion, less pain, higher quadriceps strength, greater tibial rotation, higher Marx Scale score, higher athletic confidence, higher preoperative knee self-efficacy, lower kinesiophobia and higher preoperative self-motivation.108 Müller et al111 (level B prospective cohort study) added better self-reported knee function and better hop test performance to this list.
Thomeé et al112 (level B prospective cohort study) described that there were poor results at 2 years after ACLR when testing leg muscle power and hop performance and applying an Limb Symmetry Index (LSI) of >90% to all six tests. Only 23% of patients passed when using these criteria and only 10% passed when an LSI of 95% was used.112
The overall conclusions were as follows:
Level 2: an extensive test battery should be used for determining the moment for return to play, but there are no tests or test batteries that have been tested for construct or predictive validity for return to play.23 ,27 ,28 ,108–111
Level 3: It is not clear which cut-off point of the LSI should be used for strength and hop tests.112
Return to play
Ten articles were traced about return to play: two meta-analyses,13 ,14 two systematic reviews113 ,114 and six prospective cohort studies.17 ,22 ,115–118
The meta-analysis of Ardern et al13 (level A2) included their earlier meta-analysis14 and the prospective cohort studies of Brophy et al,17 Gobbi and Francisco115 and Langford et al.22 They found that 65% of patients after ACLR returned to preinjury competitive sport level within 2 years, but only 38% remained at the same level >2 years after ACLR. Men were 1.4 times more likely to return to their preinjury sport level than women, and BPTB was 1.2 times more likely than HS.13 ,14 ,17 ,22 ,115 Laboute et al116 (level C prospective cohort study) reported 65.7% of athletes returning to preinjury sport level, while Zaffagnini et al118 reported a higher return to preinjury sport level of 71% in a group of professional soccer players 4 years after ACLR.
Several psychological factors have influence on the rehabilitation process and return to play. According to the systematic reviews of Everhart et al113 (level A2) and te Wierike et al114 (level B), a high self-efficacy, a high internal locus of control and a low level of fear are associated with a higher chance of return to play. They included the prospective cohort studies of Gobbi and Francisco,115 Langford et al22 and Thomeé et al.117
The literature concluded that:
Risk of reinjuries
Five articles about risk of reinjuries were found. These were two systematic reviews119 ,120 and three prospective cohort studies.29 ,35 ,36
The systematic reviews of Swärd et al119 and Wright et al120 (both level B) concluded that the risk of a contralateral ACL injury is higher than the risk of a first-time ACL rupture or an ACL graft rerupture. The level B prospective cohort study of Wright et al29 was included in both systematic reviews.
The level B prospective cohort studies of Hewett et al35 and Paterno et al36 support the conclusions of the systematic review of Swärd et al119 that altered neuromuscular function and biomechanics could be responsible for the risk of second ACL rupture (graft rerupture and contralateral ACL). Factors contributing could be greater hip internal rotation, the occurrence of dynamic knee valgus or less knee flexion when landing from a jump.35 ,36 ,119
Their conclusions were as follows:
Level 2: the risk of a contralateral ACL rupture (>10%) is higher than the risk of graft rerupture (about 5%) (up to 10 years after ACLR) or first-time ACL rupture.119 ,120
Level 2: altered neuromuscular function and biomechanics (greater hip internal rotation, the occurrence of dynamic knee valgus or less knee flexion during landing) after ACLR could be a risk factor for second ACL injury (graft rerupture or contralateral rupture).35 ,36 ,119
Consensus conclusion
Although there are many articles published about ACL rehabilitation, there is limited evidence for parameters that influence or predict the final result of ACLR rehabilitation and return to play. The aim of this study was to describe the process in which the KNGF evidence statement for ACL rehabilitation was developed and to present this practice guideline (see online supplementary appendix 2). The goal of the evidence statement was to describe the rehabilitation after ACLR with BPTB or HS autograft and to encourage uniformity in physical therapy treatment and the use of measurements of functional performance. The evidence statement is aimed to fill a gap between evidence and clinical practice and describes a complete protocol to rehabilitate an athlete after ACLR. The multidisciplinary approval of this evidence statement underlines the importance of a close collaboration between different professions.
Despite the fact that our evidence statement is based on information from RCTs and systematic reviews from the two most important databases, the evidence is inconclusive. Owing to this lack of scientific evidence, available background literature and a steering group consisting of ACL experts were used to develop a multidisciplinary consensus statement for an ACLR rehabilitation protocol. This consensus statement was based on three formulated questions with the following conclusions.
What should be the content of the rehabilitation protocol after ACLR?
The description of the rehabilitation protocol is divided into preoperative and postoperative rehabilitation.
Preoperative rehabilitation
Preoperative rehabilitation, also known as prehabilitation, is not usually prescribed by orthopaedic surgeons (or trauma surgeons) in the Netherlands. Previous studies showed that a preoperative full extension ROM reduces the chance for postoperative complications as arthrofibrosis.46 ,48 Moreover, a deficit in quadriceps strength of 20% or more predicts a significant strength deficit until 2 years after ACLR (level 2).41 ,121 Therefore, the steering group recommends to measure the preoperative ROM and quadriceps strength as part of the preoperative rehabilitation protocol. The steering group also advises to measure HS strength, although there is no recommendation for HS measurement in literature. Yet, there are studies that conclude that HS strength in the operated leg is still reduced compared to the non-operated leg until 2 years after ACLR.121 For this examination and possible treatment, the patient could be referred to a physical therapist to prevent a complicated or prolonged rehabilitation.
Preoperative information about walking with crutches, the early postoperative exercises and the rehabilitation process may improve a patients' self-efficacy; thus, the steering group advises to discuss these topics with patients (level 4). See also table 6 for a summary of conclusions and recommendations.
Summary of conclusions and recommendations
Postoperative rehabilitation
Good communication between the surgeon and physical therapist is of great importance. While the orthopaedic surgeon is responsible for the surgery results and techniques, the physical therapist should be leading in decision-making in rehabilitation. Therefore, the steering group advises that the orthopaedic surgeon (or trauma surgeon) informs the physical therapist about perioperative findings: graft type, menisectomy or meniscus repair, cartilage damage (location, size and grade), ligamentous injuries or complications during surgery. Also, when possible in his setting, the physical therapist should inform the surgeon about the current status of the patient preceding to every preoperative or postoperative outpatient appointment, to ensure that appropriate levels of stress are being applied to the healing tissues.122
During the first meeting of the working and steering group, it was decided to define different phases during rehabilitation after ACLR. Current literature describes time-based rehabilitation protocols that are mainly based on the remodelling process of the graft.51 Since there is still uncertainty about the time schedule of the human remodelling process, it makes more sense to incorporate functional goal-based criteria to the rehabilitation protocol.123–126 Besides, there are individual differences in neuromotor learning and flexibility after ACLR. These underline the importance of a shift from time-based rehabilitation to goal-based rehabilitation with neuromuscular goals and criteria to manage the rehabilitation process. These goals for progression to the next phase and description of interventions during each phase are based on the International Classification of Functioning, Disability and Health (WHO 2001). Our evidence statement consists of three phases (see online supplementary appendix 2) with a goal-based progression: the so-called traffic-light method of progression through phases. This is relatively new in rehabilitation, but it assures a more patient-tailored rehabilitation.118 ,127 Patients can start with the next phase only if specific goals of the previous phase are achieved and these should be confirmed with objective tests (see online supplementary appendix 2 for criteria).
The steering group advises to start rehabilitation immediate after ACLR and continue rehabilitation for 9–12 months, depending on the final return-to-work or play goals of the patient.128 This rehabilitation period is necessary to allow return to high-intensity sport or physically demanding work. This term differs from a previous ACLR rehabilitation protocol by van Grinsven et al,51 who presented a 22-week rehabilitation with four time-based phases. Recent evidence suggests that longer rehabilitation periods are needed, because most patients are not able to reach the end-rehabilitation goals in 22 weeks.129 ,130 Herbst et al131 presented a new functional performance test battery and concluded that most patients were not ready for return to play even at 8 months after ACLR. Others suggest that home-based rehabilitation is as effective as supervised rehabilitation.53 ,56 ,57 These home-based rehabilitation programmes are designed in countries where patients live too far from a physical therapist to schedule a visit a few times in a week. Important to mention is that these programmes are not designed for patients who perform high-intensity sports. Still, there is no evidence which rehabilitation period or how many appointments per week works best for return to play.
During postoperative rehabilitation, a physical therapist can use several treatment modalities, of which some are proven to be effective in literature and some are not (table 6). It is known that immediate weight bearing is safe (level 3).91 The steering group recommends that immediate weight bearing should only be tolerated if there is a correct gait pattern (if necessary with crutches) and no pain, effusion or increase in temperature when walking or shortly after walking. Cryotherapy could eventually be applied in the first postoperative week to reduce pain (level 1).103–107 The steering group suggests to start isometric quadriceps exercises in this first week for reactivating the quadriceps muscles when they provoke no pain (level 2).83 ,90 In addition, electrostimulation can be useful for re-educating voluntary contraction of the quadriceps muscles during the first postoperative weeks (level 1).63 ,93 ,94 ,98–101 When the quadriceps is reactivated, concentric and subsequently eccentric exercises should be used to replace the isometric exercises, provided that the knee does not react with effusion or (an increase in) pain. Quadriceps strength training can be performed in CKC and OKC. Concentric CKC exercises can be performed from week 2 postoperative. For OKC exercises, there should be a distinction between ACLR with a BPTB graft or a HS graft. For BPTB, OKC exercises can be started from 4 weeks postoperative in a restricted ROM of 90–45° and extra resistance is allowed, for example, at a leg extension machine (level 2). For HS, OKC exercises can also be started from 4 weeks postoperative in a restricted ROM of 90–45°, but no extra weight should be added in the first 12 weeks to prevent graft elongation (level 2).65 ,66 ROM can be increased to 90–30° in week 5, to 90–20° in week 6, to 90–10° in week 7 and to full ROM in week 8 for both graft types.51 The steering group strongly advises that neuromuscular training should be added to strength training to optimise outcome measurements (level 1).72 ,73 ,75 ,78 ,79 ,85 ,87–90
In literature about rehabilitation after ACLR, there is a lack of focus on the evaluation and training of the quality of movement as measurement of neuromuscular recovery. The relevance to focus more on the quality of movement is underlined by the fact that altered neuromuscular function and biomechanics after ACLR could be a risk factor for a second ACL injury (level 2).35 ,36 ,119 An improvement in quality of movement can be observed as an effect of motor learning. In the early phases of rehabilitation, mostly explicit motor learning is necessary, but we advocate that in the late phase of rehabilitation, more implicit motor learning strategies should be used.132 This is because implicit learning may produce more stable solutions under stress, anxiety-provoking conditions and fatigue states, especially necessary in sports.130
Which measurements and assessments can be applied to monitor progression during the rehabilitation programme and to determine outcomes at the end of rehabilitation programme?
There are no clear recommendations regarding the use of measurements for quantity (eg, strength and hop performance) and quality of movement during the postoperative rehabilitation process. The criteria to progress from phase 1 to phase 2 or from phase 2 to phase 3 are based on expert opinion (see online supplementary appendix 2). Besides the quantity and quality of movement, it is important to evaluate psychological changes during rehabilitation with an objective instrument, for example, with the Marx Scale, the Psychovitality Scale or the Knee Self-Efficacy Scale (level 2).22 ,108 ,113–115 ,117
What criteria should be used to determine the moment of return to play?
All included systematic reviews about measurements of functional performance have the same conclusion: studies are lacking objective physiological criteria at what time after ACLR return to play is allowed.27 ,31 ,32 ,108–110 There is also no conclusive evidence that any test or test battery can accurately identify athletes at high risk of reinjury. Therefore, the steering group recommends to perform an extensive test battery for quantity and quality of movement (level 2).27 ,31 ,32 ,108–111 This test battery should include at least a strength test battery and a hop test battery and measurement of quality of movement for determining the moment for return to play. An LSI of >90% could be used as a cut-off point. For pivoting/contact sports, an LSI of ≥100% is recommended (see online supplementary appendix 2).133 Qualitative scoring systems as the Jump Landing System and Landing Error Scoring System have been developed in the past few years, but it is still unclear in which manner quality of movement plays a role in the occurrence of ACL reinjuries.134–138 Therefore, prospective studies are needed to evaluate whether these scoring systems are able to measure neuromotor control and to investigate the predictive validity of those qualitative scoring systems.
Limitations
Meta-analyses and systematic reviews were included in this study. A strength is the additional weight in evidence, but a limitation is that the included meta-analyses and systematic reviews may have used other inclusion and exclusion criteria than the ones used in this study. The main discrepancy is that they did not mention the graft choice or brace-free rehabilitation in their information. We accept this limitation because many meta-analyses and systematic reviews are written about rehabilitation after ACLR and they comprise the highest level of evidence. In most cases, they give useful advice for day-to-day clinical practice and add value to the included RCTs and prospective cohort studies.
Despite the extensive literature search, our recommendations are lacking a certain specificity regarding sets, repetitions and resistance used in exercises. This is because included studies are vague in describing these parameters. However, it is extremely difficult to describe this for a population of patients, because these parameters depend on pain, effusion and level of the patient (eg, concerning type of sport and experience with strength training). We expect that every sports physical therapist is able to address the correct parameters to his individual patient, but suggest that more research is needed on this topic.
What are the findings?
Rehabilitation after anterior cruciate ligament reconstruction should consist of three phases, which are goal-based rather than time-based. The goals for progression to the next phase and description of interventions during each phase should be based on the International Classification of Functioning, Disability and Health.
An extensive test battery, including at least a strength test battery, a hop test battery and measurement of quality of movement, is needed to determine the moment of return to play.
How might it impact on clinical practice in the future?
Thirty-five per cent of athletes after anterior cruciate ligament reconstruction (ACLR) do not return to preinjury sport level within 2 years.
Closed and open kinetic chain quadriceps training can be used for regaining strength, but neuromuscular training should be added to strength training to optimise outcome measurements after ACLR.
Movement quality may affect the ACL (re)injury rate.
Acknowledgments
The authors appreciate the tremendous efforts of Erik Hendriks in the working group. The authors thank the steering group (M. Antvelink, P.A. van Beek, M. Eskes, R.P.A. Janssen, A.F. Lenssen and Th.P.H. van Thiel), and the KNGF for their contributions in realising the evidence statement.
References
Footnotes
Twitter Follow Nicky van Melick @KneeSearch
Funding The realisation of the KNGF evidence statement was funded by the KNGF.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.