Abstract
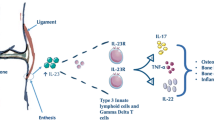
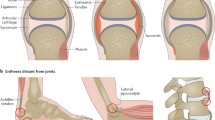
The importance of enthesitis in the pathogenesis of spondyloarthropathy (SpA) is now well recognized. Several entheses comprise more than simply the insertion site, and they are part of a complex biomechanical organ to resist shear and compression. It is also evident that tendons that wrap around bony pulleys form an integral part of joint capsules, and share, along with imaging abnormalities and histopathologic changes, common anatomic, histologic, and biomechanical features with classically defined entheses. Researchers have called these regions of tendons functional entheses. Furthermore, certain synovial joints have much in common with classic entheses—most notably those lined with fibrocartilage rather than hyaline cartilage. These observations provide a unifying anatomic basis for SpA. Enthesitis is associated with underlying osteitis, whether mechanically induced or inflammatory-related—with the extent of osteitis determined by the human leukocyte antigen-B27 gene. Until recently, there was no effective therapy for resistant enthesitis, but it is now evident that enthesitis responds well to biologic blockade with anti-tumor necrosis factor. Unraveling the pathogenic basis of enthesitis will have important implications for understanding and defining therapies in SpA.
Similar content being viewed by others
References and Recommended Reading
Brewerton DA, Caffrey H, Hart FD, et al.: Ankylosing spondylitis and HLA 27. Lancet 1973, 1:904–997.
Ball J: Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis 1971, 30:213–223.
Bywaters EGL: Pathology of spondyloarthropathies. In Spondyloarthropathies. Edited by Calin A. London: Grunne and Stratton; 1984:43–68.
Schichikawa K, Tsujimoto M, Nishioka J, et al.: Histopathology of early sacroiliitis and enthesitis in ankylosing spondylitis. In Advances in Inflammation Research, vol 9: The Spondyloarthropathies. Edited by Chen SB. New York: Raven Press; 1985:15–24.
Ball J:Articular pathology of ankylosing spondylitis. Clin Orthop 1979, 143:30–37.
McGonagle D, Gibbon W, O’Connor P, et al.:Characteristic MRI entheseal changes of knee synovitis in spondyloarthropathy. Arthritis Rheum 1998, 41:694–700.
McGonagle D, Gibbon W, Emery P:Classification of inflammatory arthritis by enthesitis. Lancet 1998, 352:1137–1140. This paper proposes that the pathogenesis of synovial joint inflammation in SpA is fundamentally different from RA. Although RA is a primary synovial immune complex-based disease, the synovitis in SpA could be secondary to enthesitis.
Benjamin M, McGonagle D: The anatomical basis for disease localization in seronegative spondyloarthropathy at entheses and related sites. J Anatomy 2001, 199:503–526. This paper draws on the anatomic, histologic, and histopathologic data, as well as imaging data, to explore the basis for disease localization in seronegative SpA. It shows how the enthesis and other sites prone to disease in SpA are functionally related, and proposes a unifying biomechanical basis for disease.
Hems T, Tillmann B:Tendon entheses of the human masticatory muscles. Anat Embryolol 2000, 202:201–208.
Benjamin M, Ralphs JR: Tendons and ligaments: an overview. Histol Histopath 1997, 12:1135–1144.
Benjamin M, Ralphs JR:Fibrocartilage in tendons and ligaments: an adaptation to compressive load. J Anatomy 1998, 193:481–494.
Benjamin M, Ralphs JR: The attachment of tendons and ligaments to bone. In Biology of the Synovial Joint. Edited by Archer CW, Caterson B, Benjamin M, Ralphs JR. Amsterdam: Harwood Academic Publishers; 1999:361–373.
Canoso JJ:Bursae, tendons and ligaments. Clin Rheum Dis 1981, 7:189–221.
Rufai A, Ralphs JR, Benjamin M:Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res 1995, 13:585–593.
Benjamin M, Ralphs JR, Shibu M, Irwin M:Capsular tissues of the proximal interphalangeal joint: normal composition and effects of Dupuytren’s disease and rheumatoid arthritis. J Hand Surgery 1993, 8B:371–376.
Lewis AR, Ralphs JR, Kneafsey B, Benjamin M: The distribution of collagens and glycosaminoglycans in the joint capsule of the proximal interphalangeal joint of the human finger. Anat Rec 1998, 250:281–291.
Kumai T, Benjamin M: Heel spur formation and the subcalcaneal enthesis of the plantar fascia in Man. J Rheumatol 2002, In press. This paper shows that heel spurs are not actually in the plantar fascia at all, but lie just beneath it. They are associated with degenerative changes in the plantar fascial enthesis that parallel those seen in articular cartilage in osteoarthritis. The spurs are, thus, interpreted as being equivalent to peripheral osteophytes in articular cartilage.
Jevtic V, Watt I, Rozman B, et al.:Distinctive radiological features of small hand joints in rheumatoid arthritis and seronegative spondyloarthritis demonstrated by contrastenhanced (Gd-DTPA) magnetic resonance imaging. Skeletal Rad 1995, 24:351–355.
Vogel KG: Fibrocartilage in tendon: a response to compressive load. In Repetitive Motion Disorders of the Upper Extremity. Edited by Gordon SL, Blair SJ, Fine LJ. Rosemont: American Academy of Orthopaedic Surgeons; 1995:205–215.
Vogel KG, Koob TJ:Structural specialization in tendons under compression. Intl Review Cytology 1989, 115:267–293.
Maksymowych WP: Ankylosing spondylitis: at the interface of bone and cartilage. J Rheumatol 2000, 27:2295–2301. This paper skillfully argues against a purely enthesitis-based model for SpA, and develops the idea that AS may be caused by fibrocartilage autoimmunity.
Katzman BM, Klein DM, Garven TC, et al.:Comparative histology of the annular and cruciform pulleys. J Hand Surgery 1999, 24B:272–274.
Sampson SP, Badalamente MA, Hurst LC, Seidman J: Pathobiology of the human A1 pulley in trigger finger. J Hand Surgery 1991, 16:714–721.
McGonagle D, Marzo-Ortega H, O’Connor P, et al.:The role of biomechanical factors and HLA-B27 on magnetic resonance imaging determined bone changes in plantar fascia enthesopathy. Arthritis Rheum 2002, 46:489–443. This paper shows that mechanically and inflammatory-induced enthesitis are associated with adjacent osteitis. The extent of osteitis is determined by the HLA-B27 gene. This has implications for defining the mechanism of disease in SpA.
Woo SL-Y, Maynard J, Butler D, et al.: Ligament, tendon, and joint capsule insertions to bone. In Injury and Repair of the Musculoskeletal Soft Tissues. Edited by Woo SL-Y, Buckwalter JA. Park Ridge: American Academy of Orthopaedic Surgeons; 1988:133–166.
Watt I: Basic differential diagnosis of arthritis. Eur Radiol 1997, 7:344–351.
Lehtinen A, Taavitsainen M, Leirisalo-Repo M:Sonographic analysis of enthesopathy in the lower extremities of patients with spondylarthropathy. Clin Exp Rheumatol 1994, 12:143–148.
D’Agostino MA, Breban M, Said-Nahal R, Dougados M: Refractory inflammatory heel pain in spondylarthropathy: a significant response to infliximab documented by ultrasound. Arthritis Rheum 2002, 46:840–841.
Francois RJ, Gardner DL, Degrave EJ, Bywaters EG:Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis. Arthritis Rheum 2000, 43:2011–2024.
Braun J, Bollow M, Neure L, et al.:Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum 1995, 38:499–505.
Bollow M, Fischer T, Reisshauer H, et al.:Quantitative analysis of sacroiliac biopsies in spondyloarthropathies: T cell and macrophages predominate in early and active sacroiliitiscellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 2000, 59:135–40.
Laloux L, Voisin MC, Allain J, et al.:Immunohistological study of entheses in spondyloarthropathy comparison in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis 2001, 60:316–21. s paper shows that inflammatory cell infiltration in the bone adjacent to insertions in SpA appears to be much more common than clinically recognized
McGonagle D, Marzo-Ortega H, O’Connor P, et al.: Histological assessment of the acute enthesitis lesion in spondyloarthropathy. Ann Rheum Dis 2002, In press. This is the first study to define the inflammatory cell infiltrate in acute enthesitis that appears to be predominantly macrophages. This has implications for understanding the mechanisms of action of biologic blockade in AS.
McGonagle D, Stockwin L, Isaacs J, Emery P: An enthesitis based model for the pathogenesis of spondyloarthropathy: additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol 2001, 28:2155–2159.
FranÇois RJ, Braun J, Khan MA:Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Curr Opin Rheumatol 2001, 13:255–264.
McGonagle D, Emery P:Enthesitis, osteitis, microbes, biomechanics, and immune reactivity in ankylosing spondylitis. J Rheumatol 2000, 27:2302–2305.
Marzo-Ortega H, Emery P, McGonagle D: The concept of disease modification in spondyloarthropathy. J Rheumatol 2002, In press.
Marzo-Ortega H, McGonagle D, O’Connor P, Emery P: Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum 2001, 44:2112–2117.
Braun J, Brandt J, Listing J, et al.: Treatment of active ankylosing spondylitis with infliximab: a randomized controlled multicenter trial. Lancet 2002, 359:1187–1193.
Poole AR:The histopathology of ankylosing spondylitis: are there unifying hypotheses? Am J Med Sciences 1998, 316:228–223.
Ahlstrom H, Feltelius N, Nyman R, Hallgren R:Magnetic resonance imaging of sacroiliac joint inflammation. Arthritis Rheum 1990, 33:1763–1769.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McGonagle, D., Benjamin, M., Marzo-Ortega, H. et al. Advances in the understanding of entheseal inflammation. Curr Rheumatol Rep 4, 500–506 (2002). https://doi.org/10.1007/s11926-002-0057-2
Issue Date:
DOI: https://doi.org/10.1007/s11926-002-0057-2