Abstract
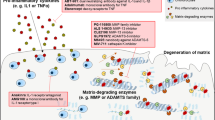
Osteoarthritis (OA) can be a progressive, disabling disease, leading to diminished quality of life, and, for over 500,000 individuals annually in the US, total joint replacement. The etiology of OA will vary among individuals, with potential roles for systemic factors (such as genetics and obesity) as well as for local biomechanical factors (such as muscle weakness, joint laxity and traumatic injury). Joint deterioration occurs over extended periods of time, and the diverse molecular mechanisms that mediate pathogenic events of early, mid and late disease are not yet fully understood. The success of biologic therapies in the treatment of rheumatoid arthritis has demonstrated that the blockade of a single dominant cytokine or regulatory molecule can prevent cartilage destruction in a complex disease, and has raised expectations that mechanism-based treatments could also be developed for patients with OA. In this review, we will address the biological mechanisms that mediate structural damage in OA and examine current targets that are candidates for disease modification. The challenges to drug development and the obstacles to disease modification strategies will also be addressed.
Key Points
-
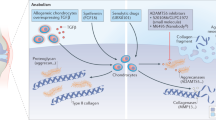
Osteoarthritis affects the three major components (bone, synovium, and cartilage) of diarthrodial joints, which produce cytokines, growth factors, proteases and other mediators that have been implicated in disease progression
-
Progressive destruction of articular cartilage is characterized by the dysregulation of anabolic and catabolic metabolic pathways; determination of these pathophysiologic processes could lead to the identification of specific targets for disease-modifying drugs
-
Currently available agents (such as glucosamine, hyaluronans, diacerein, bisphosphonates and doxycycline) have been reported to affect disease progression in osteoarthritis; however, the studies are inconclusive, and none of these agents have met regulatory criteria for structure modification
-
Inhibitors of interleukin-1, nitric-oxide synthase, bone resorption and matrix metalloproteinases are in clinical development as potential disease-modifying drugs
-
DMOAD development should be facilitated by improved imaging technology, as well as by the validation of biochemical markers of disease progression and activity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Pelletier JP et al. (2001) Osteoarthritis, an inflammatory disease: potential implications for the selection of new therapeutic agents. Arthritis Rheum 44: 1237–1247
Wieland HA et al. (2005) Osteoarthritis—an untreatable disease? Nat Rev Drug Discov 4: 331–334
Hutchinson JW et al. (1998) Dupuytren's disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J Bone Joint Surg Br 80: 907–908
Brandt KD et al. (2005) Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum 52: 2015–2025
Bettica P et al. (2002) Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum 46: 3178–3184
Hayami T et al. (2004) The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum 50: 1193–1206
Ayral X et al. (2005) Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13: 361–367
Aigner T et al. (2002) Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr Opin Rheumatol 14: 578–584
Glasson SS et al. (2005) Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434: 644–648
Attur MG et al. (2000) Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J Biol Chem 275: 40307–40315
Smith AJ et al. (2004) Extended haplotypes and linkage disequilibrium in the IL1R1–IL1A–IL1B–IL1RN gene cluster: association with knee osteoarthritis. Genes Immun 5: 451–460
Caron JP et al. (1996) Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum 39: 1535–1544
Zhang X et al. (2004) Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res 22: 742–750
Fernandes J et al. (1999) In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol 154: 1159–1169
Chevalier X et al. (2005) Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study J Rheumatol 32: 1317–1323
Chevalier X et al. (2005) Results from a double blind, placebo-controlled, multicenter trial of a single intra-articular injection of anakinra in patients with osteoarthritis of the knee. Arthritis Rheum 52 (Suppl): S507
Economides AN et al. (2003) Cytokine traps: Multi-component, high-affinity blockers of cytokine action. Nat Med 9: 47–52
Rudolphi K et al. (2003) Pralnacasan, an inhibitor of interleukin-1beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis Cartilage 11: 738–746
Kobayashi T et al. (2005) Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, reduces the progression of experimental osteoarthritis in guinea pigs. Arthritis Rheum 52: 479–487
Fukui N et al. (2003) Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am 85-A (Suppl 3): S59–S66
Clements KM et al. (2003) Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum 48: 3452–3463
Patel IR et al. (1998) TNF-alpha convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-alpha. J Immunol 160: 4570–4579
Kobayashi M et al. (2005) Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 52: 128–135
Attur MG et al. (1998) Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc Assoc Am Physicians 110: 65–72
Kaneko S et al. (2000) Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther 6: 71–79
de Hooge AS et al. (2005) Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage 13: 66–73
Merz D et al. (2003) IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol 171: 4406–4415
Yasuhara R et al. (2005) Interleukin-1beta induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J 389: 315–323
Pelletier J et al. (1999) Reduction in the structural changes of experimental osteoarthritis by a nitric oxide inhibitor. Osteoarthritis Cartilage 7: 416–418
van den Berg WB et al. (1999) Animal models of arthritis in NOS2-deficient mice. Osteoarthritis Cartilage 7: 1413–1415
Caldwell JR et al. (2000) A safety, tolerability and pharmaco-kinetic study of intra-articular recombinant human insulin-like growth factor 1 (rhIGF-1) in patients with severe osteoarthritis of the knee. Arthritis Rheum 43: S223
Haupt JL et al. (2005) Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res 23: 118–126
Food and Drug Administration [http://www.fda.gov/cder/guidance/2199dft.doc]
Dougados M et al. (2001) Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Arthritis Rheum 44: 2539–2547
Pham T et al. (2004) Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomised controlled study in symptomatic knee osteoarthritis. Ann Rheum Dis 63: 1611–1617
Reginster JY et al. (2001) Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 357: 251–256
Pavelka K et al. (2002) Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 162: 2113–2123
Mazzuca SA et al. (2002) Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 46: 1223–1227
Jubb RW et al. (2003) A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract 57: 467–474
Spector TD et al. (2005) Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial. Arthritis Res Ther 7: R625–R633
Garnero P et al. (2004) Treatment with risedronate reduced urinary CTX-II, a specific biochemical marker of cartilage type II collagen degradation, in a 24 month study of knee OA. Arthritis Rheum 50 (Suppl): S656
Buckland-Wright C et al. (2005) Risedronate protects against subchondral bone loss in OA knee patients with progressive joint space narrowing. Arthritis Rheum 52 (Suppl): S460
Carbone LD et al. (2004) The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum 50: 3516–3525
Amin AR et al. (1996) A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci 93: 14014–14019
Yu LP Jr et al. (1992) Reduction of the severity of canine osteoarthritis by prophylactic treatment with oral doxycycline. Arthritis Rheum 35: 1150–1159
Mazzuca SA et al. (2006) Comparison of quantitative and semi-quantitative indicators of joint space narrowing in subjects with knee osteoarthritis. Ann Rheum Dis 65: 64–68
Raynauld JP et al. (2002) Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum 50: 476–487
Hunter D et al. (2004) The meniscus and cartilage loss. Arthritis Rheum 50: S141
Sharif M et al. (2004) Suggestions of nonlinear or phasic progression of knee ostearthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum 50: 2479–2488
Garnero P et al. (2005) Cross-sectional association of 10 molecular markers of bone, cartilage, and synovium with disease activity and radiological joint damage in patients with hip osteoarthritis: the ECHODIAH cohort. J Rheumatol 32: 697–703
Garnero P et al. (2002) Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum 46: 2613–2624
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SB Abramson: consultant to Amgen and Abbott; Y Yusuf: investigator on clinical trials sponsored by Amgen, Abbott, Roche, and Bristol Myers Squibb.
Rights and permissions
About this article
Cite this article
Abramson, S., Attur, M. & Yazici, Y. Prospects for disease modification in osteoarthritis. Nat Rev Rheumatol 2, 304–312 (2006). https://doi.org/10.1038/ncprheum0193
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0193
This article is cited by
-
Nav1.7 as a chondrocyte regulator and therapeutic target for osteoarthritis
Nature (2024)
-
Are patients with preoperative synovitis suitable for unicompartmental knee arthroplasty? Magnetic resonance imaging evidence from a retrospective cohort study
BMC Musculoskeletal Disorders (2023)
-
Disease modification in inflammatory skin disorders: opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Crystal arthropathies and osteoarthritis—where is the link?
Skeletal Radiology (2023)
-
Past, present, and future of cartilage restoration: from localized defect to arthritis
Knee Surgery & Related Research (2022)