-
PDF
- Split View
-
Views
-
Cite
Cite
N. Georgopoulos, K. Markou, A. Theodoropoulou, P. Paraskevopoulou, L. Varaki, Z. Kazantzi, M. Leglise, A. G. Vagenakis, Growth and Pubertal Development in Elite Female Rhythmic Gymnasts, The Journal of Clinical Endocrinology & Metabolism, Volume 84, Issue 12, 1 December 1999, Pages 4525–4530, https://doi.org/10.1210/jcem.84.12.6177
Close - Share Icon Share
Optimal growth depends upon both environmental and genetic factors. Among environmental factors that could alter growth and sexual maturation are stress and intensive physical training. The influence of these factors has been documented in a variety of sports, but there is limited information on rhythmic gymnasts, who have entirely different training and performance requirements.
The study was conducted during the 13th European Championships in Patras, Greece, and included 255 female rhythmic gymnasts, aged 11–23 yr. The study included measurement of height and weight, assessment of breast and pubic hair development, estimation of body fat and skeletal maturation, and registration of menarcheal age and parental height.
Gymnasts were taller than average height for age, with mean height above and mean weight below the 50th percentile. Actual height sd score was positively correlated to weight sd score (P < 0.001), number of competitions (P = 0.01), and body mass index (BMI; P < 0.001). Predicted adult height sd score was positively correlated to weight sd score (P < 0.001) and negatively to body fat (P = 0.004).
There was a delay in skeletal maturation of 1.3 yr (P < 0.001). Pubertal development was following bone age rather than chronological age. The mean age of menarche was significantly delayed from that of their mothers and sisters (P = 0.008 and P = 0.05, respectively), was positively correlated to the intensity of training and to the difference between chronological age and bone age (P < 0.001 and P = 0.002, respectively), and was negatively correlated to body fat (P < 0.001).
In the elite female rhythmic gymnasts, psychological and somatic efforts have profound effects on growth and sexual development. Despite these aberrations, adult height is not expected to be affected.
OPTIMAL GROWTH depends upon both environmental and genetic factors (1). The final height of a child growing under favorable conditions is largely dependent on genetic predisposition (1, 2). Among environmental factors that could alter the optimal pattern of growth are stress and intensive physical training (3, 4). Sports activity may also influence pubertal development, sexual maturation, and its major event, the menarche (5–8). The influence of these factors has been documented in a variety of sports, including swimming, long distance running, and gymnastics (9–11). Rhythmic gymnasts at a high elite level are particularly prone to such alterations, as they are exposed to both intensive physical training and high levels of psychological stress. Although the effects of training and environmental factors have been well documented in the literature for artistic gymnasts, to our knowledge there is no information on rhythmic gymnasts, who have entirely different training and performance requirements.
It is well known that pubertal development, menarche, growth, and biological maturation are delayed in artistic gymnasts (12–18). It is still unclear, however, to what extent these findings may be attributed to genetic predisposing factors, to the effects of intensive training, or to the particular body composition that is characteristic of these elite athletes.
The aim of this study was to assess growth and pubertal development in young elite female rhythmic gymnasts and to determine how these factors are associated with the amount and intensity of physical training and the individual gymnast’s genetic predisposition. This study is unique in character, because all parameters were obtained on the field of competition on a certain date and place.
Subjects and Methods
The data for this study were obtained during the 13th European Rhythmic Sports Gymnastics Championships in Patras-Greece (May 22–25, 1997), under the authorization of the International Federation of Gymnastics, the European Union of Gymnastics, and the Organizing Committee of the 13th European Rhythmic Sports Gymnastics Championships. All athletes participated voluntarily under the authorization of the heads of their national delegations. The study was conducted according to article 7 of the medical organization of the official International Federation of Gymnastics competitions. The study included 255 rhythmic gymnasts, aged 11–23 yr, from 27 of 29 participating European countries. A few individuals did not participate in the medical examination due to personal reasons. Two countries refused to participate in the study because their athletes were recently subjected to medical examinations. The data were provided to us, but were not included in the study. Fifty-four percent of the athletes were juniors (age, <15 yr) and 46% seniors (age, >15 yr) in high school. The study protocol included noninvasive clinical and laboratory investigations and the completion of a questionnaire.
The clinical evaluation included height and weight measurements as well as assessment of breast and pubic hair development. Height was measured by the same physician, using a Holtain stadiometer, and registered as the mean of two consecutive measurements. Pubertal development was assessed by two female doctors, according to Tanner’s stages of breast and pubic hair development (4).
The laboratory investigation included evaluation of body composition and skeletal maturation. Body composition was assessed using a portable apparatus (Futrex 5000, Futrex, Inc., Gaithersburg, MD) that estimates percent body fat and total body water based on near infrared analysis. This technique uses the principles of light absorption and reflection to convert electromagnetic radiation, transmitted and reflected through subject’s biceps, to optical density measurements used to calculate percent body fat. The accuracy and precision of the near infrared technique has been validated to be equivalent to the standard methods of body composition assessment by skinfold measurements and bioimpedance assessments (19, 20). Skeletal maturation was assessed from x-ray of the left hand and wrist taken in a separate room under full body protection from radioactivity. The radiographs were evaluated blindly by two doctors, and bone age was estimated according to Greulich-Pyle standards (21). All x-rays with a difference in bone age estimation greater than 6 months were reevaluated blindly by two radiologists. There was no occasion where, after the second reading, difference in skeletal age estimation persisted. For those athletes whose radiographs showed complete skeletal maturation (bone age, >18 yr), the measured actual height was considered to be the adult height. Prediction of adult height was estimated using the Bayley-Pinneau method, based on height and bone age, as assessed using Greulich-Pyle standards (22).
At the end of clinical and laboratory evaluation, all athletes were asked to complete a questionnaire that included both personal and family data. Personal data included the age of menarche, the onset and intensity of training (hours per week), and the number of competitions per yr. Data from family history included father’s and mother’s heights, and mother’s and sisters’ ages of menarche.
The reported target height (TH) was estimated in centimeters, using the midparental height as an index of genetic predisposition to adult height. The equation used for reported target height determination is: TH = (father’s height − 13 + mother’s height)/2.
For statistical evaluation, height and weight were expressed as the sd score of the mean height and weight for age, using Tanner’s standards (23). The sd score was also calculated for reported target height and predicted adult height.
Statistics
The Pearson correlation test, with two-tailed test of significance, was used to study relations between the sd scores of height, menarche, and genetic, metabolic, and sport-related factors. Student’s t test was used to study the differences between gymnasts and their mothers’ and sisters’ ages of recalled menarche. All correlations with a critical value of P< 0.05 were considered significant.
Results
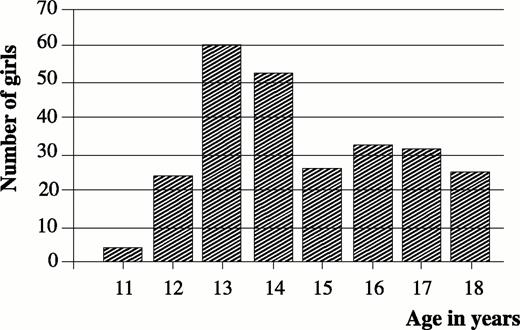
The age distribution of examined gymnasts ranged from 11–23 yr. The higher percentage was between 13–15 yr (54.5%), with a peak at the age of 13 yr (23.5%; Fig. 1).
Anthropometric characteristics
The mean values and sd scores for collected and derived data are shown in Table 1A. For 16 athletes who showed complete skeletal maturation on radiographs, their actual measured height was considered as adult height. The mean values and sd scores for adult height and for the difference between adult height sd score and reported target height sd score are shown in Table 1B.
Collected and derived data of 16 rhythmic gymnasts who had reached adult height
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 18.43 | 2.09 |
| Ht (cm) | 168.2 | 5.20 |
| Wt (kg) | 52.4 | 5.10 |
| Derived data | ||
| Adult ht sd score | 1.00 | 0.87 |
| Target ht sd score | 0.84 | 0.68 |
| Adult ht sd score minus target ht sd score | 0.23 | 0.60 |
| Wt sd score | −0.47 | 0.48 |
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 18.43 | 2.09 |
| Ht (cm) | 168.2 | 5.20 |
| Wt (kg) | 52.4 | 5.10 |
| Derived data | ||
| Adult ht sd score | 1.00 | 0.87 |
| Target ht sd score | 0.84 | 0.68 |
| Adult ht sd score minus target ht sd score | 0.23 | 0.60 |
| Wt sd score | −0.47 | 0.48 |
Collected and derived data of 16 rhythmic gymnasts who had reached adult height
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 18.43 | 2.09 |
| Ht (cm) | 168.2 | 5.20 |
| Wt (kg) | 52.4 | 5.10 |
| Derived data | ||
| Adult ht sd score | 1.00 | 0.87 |
| Target ht sd score | 0.84 | 0.68 |
| Adult ht sd score minus target ht sd score | 0.23 | 0.60 |
| Wt sd score | −0.47 | 0.48 |
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 18.43 | 2.09 |
| Ht (cm) | 168.2 | 5.20 |
| Wt (kg) | 52.4 | 5.10 |
| Derived data | ||
| Adult ht sd score | 1.00 | 0.87 |
| Target ht sd score | 0.84 | 0.68 |
| Adult ht sd score minus target ht sd score | 0.23 | 0.60 |
| Wt sd score | −0.47 | 0.48 |
Collected and derived data of examined rhythmic gymnasts
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 14.73 | 2.12 |
| Bone age (yr) | 13.55 | 1.92 |
| Ht (cm) | 160.4 | 7.40 |
| Mother’s ht (cm) | 165.5 | 5.81 |
| Father’s ht (cm) | 177.6 | 6.68 |
| Wt (kgr) | 42.0 | 7.37 |
| BMI | 16.26 | 1.82 |
| Body fat (%) | 16.1 | 4.07 |
| Onset of training (yr) | 6.82 | 1.92 |
| No. of competitions | 6.58 | 3.04 |
| Intensity of training (h/week) | 29.14 | 15.35 |
| Derived data | ||
| Ht sd score | 0.22 | 1.03 |
| Reported target ht (cm) | 165.5 | 5.08 |
| Target sd score | 0.48 | 0.84 |
| Predicted ht | 167.5 | 5.59 |
| Predicted ht sd score | 0.88 | 0.93 |
| Predicted ht sd score minus target ht sd score | 0.11 | 0.93 |
| Wt sd score | −1.0 | 0.63 |
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 14.73 | 2.12 |
| Bone age (yr) | 13.55 | 1.92 |
| Ht (cm) | 160.4 | 7.40 |
| Mother’s ht (cm) | 165.5 | 5.81 |
| Father’s ht (cm) | 177.6 | 6.68 |
| Wt (kgr) | 42.0 | 7.37 |
| BMI | 16.26 | 1.82 |
| Body fat (%) | 16.1 | 4.07 |
| Onset of training (yr) | 6.82 | 1.92 |
| No. of competitions | 6.58 | 3.04 |
| Intensity of training (h/week) | 29.14 | 15.35 |
| Derived data | ||
| Ht sd score | 0.22 | 1.03 |
| Reported target ht (cm) | 165.5 | 5.08 |
| Target sd score | 0.48 | 0.84 |
| Predicted ht | 167.5 | 5.59 |
| Predicted ht sd score | 0.88 | 0.93 |
| Predicted ht sd score minus target ht sd score | 0.11 | 0.93 |
| Wt sd score | −1.0 | 0.63 |
Collected and derived data of examined rhythmic gymnasts
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 14.73 | 2.12 |
| Bone age (yr) | 13.55 | 1.92 |
| Ht (cm) | 160.4 | 7.40 |
| Mother’s ht (cm) | 165.5 | 5.81 |
| Father’s ht (cm) | 177.6 | 6.68 |
| Wt (kgr) | 42.0 | 7.37 |
| BMI | 16.26 | 1.82 |
| Body fat (%) | 16.1 | 4.07 |
| Onset of training (yr) | 6.82 | 1.92 |
| No. of competitions | 6.58 | 3.04 |
| Intensity of training (h/week) | 29.14 | 15.35 |
| Derived data | ||
| Ht sd score | 0.22 | 1.03 |
| Reported target ht (cm) | 165.5 | 5.08 |
| Target sd score | 0.48 | 0.84 |
| Predicted ht | 167.5 | 5.59 |
| Predicted ht sd score | 0.88 | 0.93 |
| Predicted ht sd score minus target ht sd score | 0.11 | 0.93 |
| Wt sd score | −1.0 | 0.63 |
| Variable . | Mean . | sd . |
|---|---|---|
| Collected data | ||
| Age (yr) | 14.73 | 2.12 |
| Bone age (yr) | 13.55 | 1.92 |
| Ht (cm) | 160.4 | 7.40 |
| Mother’s ht (cm) | 165.5 | 5.81 |
| Father’s ht (cm) | 177.6 | 6.68 |
| Wt (kgr) | 42.0 | 7.37 |
| BMI | 16.26 | 1.82 |
| Body fat (%) | 16.1 | 4.07 |
| Onset of training (yr) | 6.82 | 1.92 |
| No. of competitions | 6.58 | 3.04 |
| Intensity of training (h/week) | 29.14 | 15.35 |
| Derived data | ||
| Ht sd score | 0.22 | 1.03 |
| Reported target ht (cm) | 165.5 | 5.08 |
| Target sd score | 0.48 | 0.84 |
| Predicted ht | 167.5 | 5.59 |
| Predicted ht sd score | 0.88 | 0.93 |
| Predicted ht sd score minus target ht sd score | 0.11 | 0.93 |
| Wt sd score | −1.0 | 0.63 |
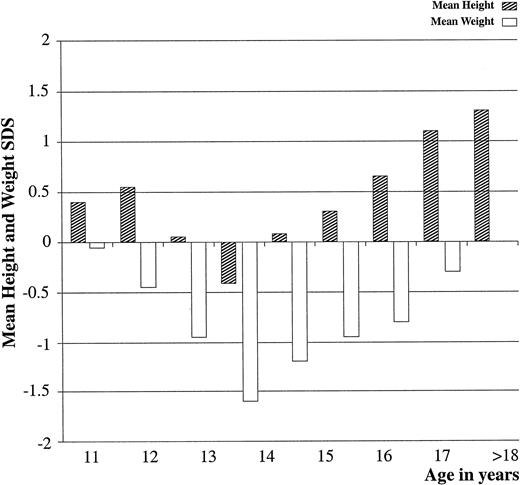
The height sd score and weight sd score for each age group from 11–23 yr of age are shown in Fig. 2. It is obvious that gymnasts were taller than the average height for age, with mean heights on or above the 50th percentile. However, gymnasts were lighter than average, with mean weight for age below the 50th percentile. In general, the sd score for height followed a sigmoid type of curve, whereas the weight curve was a negative parabola. This was particularly evident at age 13, 14, and 15 yr, with a peak at age 14 yr. At this age, the sd score for height was negative, accompanied by the most impressive negative score for weight. The mean values and sd scores for chronological and bone age are shown in Table 1A. There was a delay of 1.3 yr in skeletal maturation, which is statistically significant (P < 0.001).
Sexual maturation
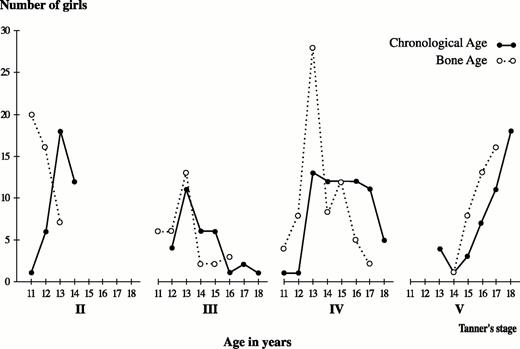
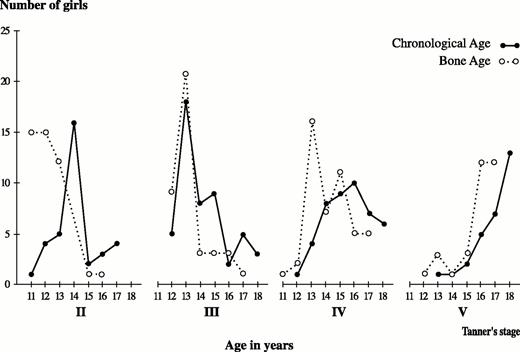
The distribution of pubertal development, according to chronological and bone age, is shown in Fig. 3 for pubic hair development (Tanner’s stages I–V) and in Fig. 4 for breast development (Tanner’s stages I–V). It is known that pubertal development follows bone age rather than chronological age (24). The majority of athletes had a bone age more advanced during the early stages of puberty up to the age of 14 yr. After the age of 14 yr, there was a gradual normalization of this difference, which disappeared when puberty was fully accomplished. As far as the onset of puberty for groups over 14 yr of age, the majority of athletes were following the chronological rather than the bone age.
Relationship of pubic hair development to chronological and bone ages in rhythmic gymnasts. For each stage of pubic hair development (Tanner stages II–V), the number of girls is shown on the vertical axis, and age in years is shown on the horizontal axis. For all stages of pubic hair development, bone age (open circles) precedes chronological age (dark circles) up to the age of 14 yr, but thereafter chronological age predominates.
Relationship of breast development to chronological and bone ages in rhythmic gymnasts. For each stage of breast development (Tanner stages II–V), the number of girls is shown on the vertical axis, and age in years is shown on the horizontal axis. For all stages of breast development, bone age (open circles) precedes chronological age (darkcircles) up to the age of 14 yr, but thereafter chronological age predominates.
The mean age of recalled menarche was 14.3 ± 1.46 yr. It is to be noted that only 81 of 255 examined gymnasts (32%) reported menarche, and of those still amenorrheic, 20% were older than 15 yr of age. Thus, the final mean age of recalled menarche is expected to be much higher than the present recorded one.
The mothers’ mean age of menarche was identical to the sisters of the gymnasts (13.7 ± 1.5 yr). However, the gymnasts’ mean age of menarche (14.3 ± 1.5 yr) was significantly delayed from that of their mothers’ and sisters’ (P = 0.008; t = 2.68; n = 141, and P = 0.05; t = 1.95; n = 45, respectively).
Relationships
To evaluate associations between the sd scores of height and genetic, metabolic, and sport-related factors that could influence growth, correlation coefficients were calculated between actual height sd score, target height sd score, predicted adult height sd score, difference between predicted adult height sd score and target height sd score, difference between chronological age and bone age, BMI, body fat, onset of training, number of competitions per yr, and intensity of training. These correlations are presented in Table 2.
Actual, predicted, and target height sd score: correlation coefficients
| . | Actual ht sd score . | Predicted adult ht sd score . | Predicted adult ht sd score − target ht sd score . |
|---|---|---|---|
| Wt sd score | n = 242, r = 0.73, P < 0.001 | n = 186, r = 0.33, P < 0.001 | n = 163, r = 0.25, P = 0.001 |
| BMI | n = 255, r = 0.36, P < 0.001 | n = 198, r = 0.08, P = 0.25 | n = 173, r = −0.04, P = 0.50 |
| Body fat | n = 253, r = −0.10, P = 0.08 | n = 127, r = −0.21, P = 0.004 | n = 172, r = −0.21, P = 0.05 |
| Onset of training | n = 207, r = 0.04, P = 0.57 | n = 156, r = −0.06, P = 0.44 | n = 150, r = −0.19, P = 0.01 |
| No. of competitions | n = 226, r = 0.16, P = 0.01 | n = 175, r = 0.05, P = 0.50 | n = 172, r = −0.10, P = 0.20 |
| Training h/weeks | n = 233, r = −0.03, P = 0.60 | n = 182, r = 0.05, P = 0.57 | n = 150, r = 0.12, P = 0.12 |
| . | Actual ht sd score . | Predicted adult ht sd score . | Predicted adult ht sd score − target ht sd score . |
|---|---|---|---|
| Wt sd score | n = 242, r = 0.73, P < 0.001 | n = 186, r = 0.33, P < 0.001 | n = 163, r = 0.25, P = 0.001 |
| BMI | n = 255, r = 0.36, P < 0.001 | n = 198, r = 0.08, P = 0.25 | n = 173, r = −0.04, P = 0.50 |
| Body fat | n = 253, r = −0.10, P = 0.08 | n = 127, r = −0.21, P = 0.004 | n = 172, r = −0.21, P = 0.05 |
| Onset of training | n = 207, r = 0.04, P = 0.57 | n = 156, r = −0.06, P = 0.44 | n = 150, r = −0.19, P = 0.01 |
| No. of competitions | n = 226, r = 0.16, P = 0.01 | n = 175, r = 0.05, P = 0.50 | n = 172, r = −0.10, P = 0.20 |
| Training h/weeks | n = 233, r = −0.03, P = 0.60 | n = 182, r = 0.05, P = 0.57 | n = 150, r = 0.12, P = 0.12 |
Actual, predicted, and target height sd score: correlation coefficients
| . | Actual ht sd score . | Predicted adult ht sd score . | Predicted adult ht sd score − target ht sd score . |
|---|---|---|---|
| Wt sd score | n = 242, r = 0.73, P < 0.001 | n = 186, r = 0.33, P < 0.001 | n = 163, r = 0.25, P = 0.001 |
| BMI | n = 255, r = 0.36, P < 0.001 | n = 198, r = 0.08, P = 0.25 | n = 173, r = −0.04, P = 0.50 |
| Body fat | n = 253, r = −0.10, P = 0.08 | n = 127, r = −0.21, P = 0.004 | n = 172, r = −0.21, P = 0.05 |
| Onset of training | n = 207, r = 0.04, P = 0.57 | n = 156, r = −0.06, P = 0.44 | n = 150, r = −0.19, P = 0.01 |
| No. of competitions | n = 226, r = 0.16, P = 0.01 | n = 175, r = 0.05, P = 0.50 | n = 172, r = −0.10, P = 0.20 |
| Training h/weeks | n = 233, r = −0.03, P = 0.60 | n = 182, r = 0.05, P = 0.57 | n = 150, r = 0.12, P = 0.12 |
| . | Actual ht sd score . | Predicted adult ht sd score . | Predicted adult ht sd score − target ht sd score . |
|---|---|---|---|
| Wt sd score | n = 242, r = 0.73, P < 0.001 | n = 186, r = 0.33, P < 0.001 | n = 163, r = 0.25, P = 0.001 |
| BMI | n = 255, r = 0.36, P < 0.001 | n = 198, r = 0.08, P = 0.25 | n = 173, r = −0.04, P = 0.50 |
| Body fat | n = 253, r = −0.10, P = 0.08 | n = 127, r = −0.21, P = 0.004 | n = 172, r = −0.21, P = 0.05 |
| Onset of training | n = 207, r = 0.04, P = 0.57 | n = 156, r = −0.06, P = 0.44 | n = 150, r = −0.19, P = 0.01 |
| No. of competitions | n = 226, r = 0.16, P = 0.01 | n = 175, r = 0.05, P = 0.50 | n = 172, r = −0.10, P = 0.20 |
| Training h/weeks | n = 233, r = −0.03, P = 0.60 | n = 182, r = 0.05, P = 0.57 | n = 150, r = 0.12, P = 0.12 |
The actual height sd score was positively correlated to weight sd score (P < 0.001), to the number of competitions per yr (P = 0.01), and to BMI (P < 0.001). The target height sd score was positively correlated to both actual height sd score (P < 0.001) and predicted adult height sd score (P < 0.001). The weight sd score was positively correlated to actual height sd score (P < 0.001), to predicted adult height sd score (P < 0.001), and to the difference between predicted adult height sd score and target height (P = 0,001). BMI was positively correlated to actual height sd score (P < 0.001). Body fat was negatively correlated to predicted adult height sd score (P = 0.004) as well as to the difference between predicted adult height sd score and final height sd score (P = 0.05). The onset of training was negatively correlated to the difference between predicted adult height sd score and adult height sd score (P = 0.01).
All correlation coefficients concerning gymnasts’ age of recalled menarche are presented in Table 3.
Gymnasts’ age of menarche: correlation coefficients
| Parameter . | n . | r . | P value . |
|---|---|---|---|
| Body fat | 80 | −0.36 | <0.001 |
| Difference CA− BA | 65 | 0.37 | 0.002 |
| Training h/week | 78 | 0.39 | <0.001 |
| No. of competitions | 75 | −0.14 | 0.24 |
| Onset of training | 75 | −0.07 | 0.50 |
| Wt sd score | 71 | −0.09 | 0.45 |
| BMI | 81 | −0.02 | 0.70 |
| Parameter . | n . | r . | P value . |
|---|---|---|---|
| Body fat | 80 | −0.36 | <0.001 |
| Difference CA− BA | 65 | 0.37 | 0.002 |
| Training h/week | 78 | 0.39 | <0.001 |
| No. of competitions | 75 | −0.14 | 0.24 |
| Onset of training | 75 | −0.07 | 0.50 |
| Wt sd score | 71 | −0.09 | 0.45 |
| BMI | 81 | −0.02 | 0.70 |
CA, Chronological age; BA, bone age.
Gymnasts’ age of menarche: correlation coefficients
| Parameter . | n . | r . | P value . |
|---|---|---|---|
| Body fat | 80 | −0.36 | <0.001 |
| Difference CA− BA | 65 | 0.37 | 0.002 |
| Training h/week | 78 | 0.39 | <0.001 |
| No. of competitions | 75 | −0.14 | 0.24 |
| Onset of training | 75 | −0.07 | 0.50 |
| Wt sd score | 71 | −0.09 | 0.45 |
| BMI | 81 | −0.02 | 0.70 |
| Parameter . | n . | r . | P value . |
|---|---|---|---|
| Body fat | 80 | −0.36 | <0.001 |
| Difference CA− BA | 65 | 0.37 | 0.002 |
| Training h/week | 78 | 0.39 | <0.001 |
| No. of competitions | 75 | −0.14 | 0.24 |
| Onset of training | 75 | −0.07 | 0.50 |
| Wt sd score | 71 | −0.09 | 0.45 |
| BMI | 81 | −0.02 | 0.70 |
CA, Chronological age; BA, bone age.
The age of recalled menarche was correlated positively to the intensity of training (P < 0.001) and to the difference between chronological age and bone age (P = 0.002) and negatively to body fat (P < 0.001).
Discussion
We found that elite female rhythmic gymnasts exhibit a specific pattern of growth and pubertal maturation. Skeletal maturation was markedly delayed. Although there is a discrepancy in bone age estimation depending on the methodology used (Tanner’s method of bone age estimation usually yields older ages than Greulich-Pyle’s method) (25), there is a general agreement that bone age is moderately, but significantly, delayed in gymnasts. The delay in skeletal maturation is probably multifactorial (13–18, 25, 26). Low serum concentrations of sex steroids due to a delay in pubertal development (27), lower GH secretion, or a disturbance in insulin-like growth hormone homeostasis (28, 29) could alter the hormonal control of growth. Intensive physical training, chronic psychological stress, and modifications in nutritional factors resulting in inadequate energy intake relative to energy output (30, 31) are well known factors. Nevertheless, overuse lesions of growth plates, especially in the lower limbs, could add an additional end-organ effect (3).
The finding of a delay in both breast and pubic hair development in rhythmic gymnasts in our study is in agreement with previous reports (7, 14, 25). Their phenotype (taller but also lighter than average) is not atypical for young women with constitutional delay of puberty. Their pubertal development seems to correlate better to their bone age rather than to their chronological age. In fact, rhythmic gymnasts represent a select group of girls who have been exposed to high intensity training since preadolescence. During the expected time of puberty they remain in maximal sports activity and are highly motivated to maintain low body weight. Delayed puberty is the result of hypothalamic dysfunction due to extensive physical training, stress, and/or malnutrition, leading to inappropriately low secretion of gonadotropins (23, 30).
The combined effects of the delay in skeletal and sexual maturation upon growth are evident in the gymnasts who are 13–15 yr of age. These athletes were well below average as far as height, weight, and sexual maturation due to the effects of stress induced by intense training. This is strengthened by the fact that rhythmic gymnasts, due to some degree of self selection into these sports, are usually taller and thinner compared to their normal counterparts (12–18, 25, 30, 32, 33). Because of the delay in pubertal development, the growth spurt is observed later, with an adequate recovery of growth potential.
Although moderate exercise has a stimulating effect on growth, intensive physical training represents a chronic stress capable of attenuating growth. It appears that the intensity and duration of training are more important than the type of training. There is no direct evidence that physical activity could affect adult height in female rhythmic gymnasts. In previous reports (14, 15, 26, 32), the adult height of artistic gymnasts remained appropriate for the reported target height regardless of the method used to estimate predicted adult height. A previous prospective study (33) provided evidence of a reduction of growth potential and a decrease in mean height predictions with time in a smaller group of artistic gymnasts. However, in another study by the same group (25) it was reported that the predicted adult height was not reduced in artistic gymnasts, which demonstrates the inherent inaccuracy of height predictions. Although these data are highly informative, no definite conclusions should be made unless adult height has been attained. It should be noted that in our study the adult height sd score of our smaller group of gymnasts who have completed linear growth was higher than the predicted adult height sd score of the whole study group, a finding arguing against an overestimation of our prediction of adult height. Nevertheless, the positive difference between adult height sd score and target height sd score demonstrates that in athletes who have already attained full skeletal maturation under intensive physical training, adult height is not affected.
The age of menarche is determined by both genetic and environmental factors. Highly intense physical training, chronic stress, nutritional factors, low body weight, and/or low body fat are established factors that could alter menstrual function (34–37). Late menarche is a common finding among athletes. This observation has been documented in several sports, including gymnastics (5–12, 14, 25, 38). It is still a debate among investigators, whether this delay is attributed to genetic predisposition and consequent preselection or to the effect of early-onset intensive physical training. We found that recalled menarche of elite female rhythmic gymnasts was significantly delayed compared to that of their mothers and sisters. It should be emphasized that the age of menarche is remarkably underestimated in our study, as 68% of the gymnasts reported no menarche. Considering the identical age of recalled menarche between gymnasts’ mothers and sisters, these data demonstrate that within gymnasts’ families, genetic predisposition is disrupted for trained gymnasts and preserved for their nontrained sisters.
Previous studies suggested that delayed menarche in gymnasts may be related to high selection (11, 14, 25, 38). According to Malina’s two-part hypothesis, delayed menarche in athletes is the result of combined biological selective factors and social factors (39). Athletes from families with a genetic predisposition toward late menarche are more likely to be successful in sports such as gymnastics, where late maturation may favor performance. Our data and the results of other investigators (7, 8, 10, 12, 40) provide evidence to support the second part of Malina’s hypothesis. The fact that the athletes’ sisters’ menarche was identical to that of their mothers attenuates the biological selection of the athletes and favors the social factors. According to Frisch’s theory (41, 42), a minimum percentage of body fat is absolutely necessary for the initiation of menstruation. More recently, leptin, an adipocyte-derived hormone, has been implicated as an important signal responsible for the initiation of sexual maturation (43). Previous studies have implicated intense training as a causative factor for menarcheal delay (10, 11, 40). The stress of training and competition has a well known inhibitory effect on the hypothalamic control of the reproductive axis (34, 36, 44). In our study, diminished body fat, delayed skeletal maturation, and intensive physical training have been shown to correlate to gymnasts’ age of recalled menarche. These findings support the hypothesis that it is elite sports’ activity that leads to the disruption of genetic predisposition resulting in the retardation of menarche.
In conclusion, the results of our study demonstrate the profound effects of the psychological and somatic effort to become an elite rhythmic gymnast on growth, skeletal maturation, pubertal development, and menarche. Although the adult height is expected not to be affected in these athletes, the above-mentioned aberrations in growth and pubertal development must be considered for better support of these exceptional and highly motivated individuals.
Acknowledgements
We express our grateful thanks to Mr. Klaus Lotz, President of the European Union of Gymnastics, and to Mr. Dimitris Dimitropoulos, President of the Hellenic Gymnastics Federation, who provided all necessary facilities and greatly encouraged the initiation of the present study. We are indebted to Dr. Dan Benardot, Associate Dean for Research of the College of Health and Human Sciences of Georgia State University, for critically reviewing the manuscript as well as for his substantial suggestions and insightful discussions.
President of the International Federation of Gymnastics Medical Committee.
Warren MP.
Theintz G, Torresani T, Bishof P, Weiss U, Sizonenko PC. 1993 Effects of physical exercise on growth and pubertal development. In: Muller EE, Cachi D, Laateeli V, eds. Growth hormone and somatomedins during lifespan. Springer-Verlag Berlin Heidelburg; 218–229.
Smit PJ.
Lukaski HL.
Tanner JM.
Warren M.
Malina RM.