Article Text
Abstract
Ice is commonly used after acute muscle strains but there are no clinical studies of its effectiveness. By comparison, there are a number of basic scientific studies on animals which show that applying ice after muscle injury has a consistent effect on a number of important cellular and physiological events relating to recovery. Some of these effects may be temperature dependant; most animal studies induce significant reductions in muscle temperature at the injury site. The aim of this short report was to consider the cooling magnitudes likely in human models of muscle injury and to discuss its relevance to the clinical setting. Current best evidence shows that muscle temperature reductions in humans are moderate in comparison to most animal models, limiting direct translation to the clinical setting. Further important clinical questions arise when we consider the heterogenous nature of muscle injury in terms of injury type, depth and insulating adipose thickness. Contrary to current practice, it is unlikely that a ‘panacea’ cooling dose or duration exists in the clinical setting. Clinicians should consider that in extreme circumstances of muscle strain (eg, deep injury with high levels of adipose thickness around the injury site), the clinical effectiveness of cooling may be significantly reduced.
Statistics from Altmetric.com
Acute muscle injury commonly occurs in recreational and professional sports.1,–,3 Following expert consensus,4 ice application is still recommended in the early management of muscle strain. Recent clinical guidelines on acute soft tissue injury management by the Association of Chartered Physiotherapists in Sport and Exercise Medicine (ACPSM)5 found that human studies of ice on muscle injury are distinctly lacking (ACPSM). By comparison, there was an abundance of evidence derived from basic scientific models; these consistently demonstrate that ice can influence key cellular and physiological events associated with injury recovery. The effects include decreased cellular metabolism,6,–,8 altered white cell activity within the vasculature,9,–,12 reduced muscle necrosis11 and apoptosis.12
It is anticipated that ice (or cryotherapy in general) achieves its clinical effect by cooling injured tissue. Animal models report muscle temperatures as low as 10°C11 after ice application. Such large reductions concur with basic scientific theory that decreasing tissue temperatures to 5–15°C optimally reduces cellular metabolism and therefore limits secondary ischaemic and enzymatic damage (secondary injury) in the early stages of injury.6 In comparison to other soft tissues, muscle is particularly sensitive to ischaemic conditions and may therefore be most at risk of secondary injury. The aim of this short report was to consider the cooling magnitudes likely in human models of muscle injury and discuss its relevance to the clinical setting.
Although there have been a significant number of investigations into intramuscular temperature reductions associated with cryotherapy on humans, none have included injured subjects. Most healthy models have failed to reduce human muscle tissue below 25°C, despite cooling durations of up to 50 min.5 To our knowledge, the lowest intramuscular temperature reported in human participants is approximately 21°C.13 This was achieved after 20 min of treatment with crushed ice, but was based on superficial intramuscular temperature (1-cm depth) and used a healthy sample with very low levels of adipose tissue thickness. Although the exact therapeutic temperature reduction needed to ensure ice's benefits has not been fully determined, best available evidence suggests that the magnitude and depth of cooling are important. Table 1 summarises fundamental differences between animal- and clinical-based research in this area, in terms of cooling dose, trial design and research logistics.
Cooling and muscle injury: fundamental differences between animal- and clinical-based research
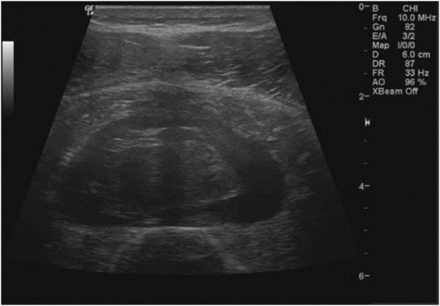
Further important questions arise when we consider the heterogenous nature of soft tissue injury in a clinical setting, particularly those involving muscle. As an example, figures 1 and 2 show ultrasonographic imaging (GE: LOGIQ e) of a convenience sample of Grade 2 muscle strains presenting to our clinic with similar clinical history. Imaging was undertaken in the early stages after injury by an experienced clinician to quantify important factors which might influence cooling efficacy at the injury site. Both the figures show low levels of adipose tissue thickness around the injury site ranging from 0.4 cm (figure 2) to 1 cm (figure 1). Both the figures represent athletic subjects, however, adipose thickness will vary according to body part or across individuals. Higher levels of adipose thickness could significantly affect the cooling rate; it has been estimated that a 1-cm increase changes cooling rate (at 1-cm intramuscular depth) from 0.72 to 0.45°C/min in healthy subjects.13 Of note, there is a significant variation across figures 1 and 2 in terms of injury depth. The injury site in figure 2 is approximately 2 cm deeper than in figure 1. Healthy models show that it is very difficult to induce large cooling magnitudes at deep target tissues; indeed, increasing the intramuscular depth by 2 cm could limit final temperature reduction by up to 8°C.13 Van't Hoff's law states that every 10°C reduction in tissue temperature equates to a two- to three-fold decrease in cellular metabolism. Therefore, a change in the depth of the injury site by 2 cm could be clinically important, particularly if our main rationale is to prevent secondary injury damage through local reductions in tissue metabolism.
Grade 2 medial gastrocnemius strain (longitudinal image: left = proximal). Depth of fibre disruption: ∼1–2 cm below the skin surface. Adipose thickness: ∼0.9 cm.
Grade 2 vastus intermedialis strain (cross-sectional image). Depth of fibre disruption: ∼2.5–5 cm below the skin surface. Adipose thickness: ∼0.4 cm.
Ice remains popular for acute muscle strain yet the evidence for its use is based on animal models which are not fully applicable to the clinical setting. Furthermore, few clinicians may consider factors such as the depth of the ‘damaged target tissue’ before initiating treatment. Recommendations on ice dosage remain anecdotal and usually do not change based on the circumstances of injury. Soft tissue injuries are clearly heterogeneous and it may be erroneous to continue to recommend a perennial or ‘panacea’ cooling dose. We must also consider that in circumstances of deep muscle injury with substantial surrounding adipose, the clinical effectiveness of cooling may be significantly reduced.
Future research into the effectiveness of ice and muscle injury is essential. Treatment dosage should potentially be developed based on injury depth, and should be factored into future clinical trial design. There are many new emerging practices for muscle injury management such as autologous blood products like platelet-rich plasma (PRP), and various other wet injections. Interestingly, there are already more controlled studies for platelet-rich technologies and soft tissue injury than for ice, compression or elevation. International Olympic Committee consensus is to proceed with caution in the use of PRP,14 and large randomised studies are clearly needed; perhaps one advantage of using PRP or other invasive wet injection after muscle injury is that they ensure direct administration of the ‘active’ agent at the injury site.
What is already known on this topic
Animal models show that ice has a consistent effect on important cellular and physiological events relating to recovery after muscle injury. Ice application is a common clinical recommendation after acute muscle strains but there are no clinical studies of its effectiveness.
What this study adds
Muscle temperature reductions in humans are moderate in comparison to most animal models. It is also unlikely that a ‘panacea’ cooling dose exists in the clinical setting. Factors such as injury depth and adipose thickness may significantly influence the clinical effectiveness of cooling.
References
Footnotes
-
Competing interests None.
-
Provenance and peer review Not commissioned; externally peer reviewed.