Article Text
Abstract
Nitric oxide (NO) is a small free radical generated by a family of enzymes, the nitric oxide synthases (NOSs). Following injury to a tendon, NO is induced by all three isoforms of NOS and NOS activity is also upregulated in tendinopathy. In animal models when NOS activity is inhibited by competitive inhibitors of NOS, tendon healing is reduced. When additional NO is added, tendon healing is enhanced. In humans, in three randomised clinical trials, we have shown that NO delivered via a transdermal patch enhances the subjective and objective recovery of patients with tennis elbow, Achilles tendinosis and supraspinatus tendinosis.
- NO, nitric oxide
- NOS, nitric oxide synthase
- bNOS, brain NOS
- eNOS, endothelial NOS
- GTN, glyceryl trinitrate
- iNOS, inducible NOS
Statistics from Altmetric.com
- NO, nitric oxide
- NOS, nitric oxide synthase
- bNOS, brain NOS
- eNOS, endothelial NOS
- GTN, glyceryl trinitrate
- iNOS, inducible NOS
Nitric oxide (NO) is a small soluble gas synthesised by a family of enzymes, the nitric oxide synthases (NOSs). Like other free radicals, in large doses NO is toxic. In smaller, physiological doses, however, it acts as a messenger molecule.
The family of NOSs consists of three isoforms regulated by a number of cofactors. eNOS (originally found in endothelial cells1) and bNOS (originally identified in brain and neuronal tissue2) are constitutive, low-output isoforms important in blood pressure regulation and memory. Inducible NOS (iNOS) is a high-output isoform important in host defence.3,4
NOS ACTIVITY FOLLOWING TENDON INJURY
There is little NOS activity in a normal, uninjured tendon. Following division, however, we found a significant (approximately fivefold) increase in NOS activity within the healing tendon in rat Achilles5 and supraspinatus tendons. The activity peaks at day 7 and returns to baseline at day 14. This activity was inhibitable by an NOS inhibitor (NG-monomethyl-l-arginine). All three NOS isoforms were expressed following rat Achilles tendon division. Four days after injury, there were increases in the steady-state levels of mRNA and protein for all 3 NOS isoforms, with peaks for iNOS (23-fold increase) at days 4 and 7, eNOS (24-fold increase) at day 7 and bNOS (7-fold increase) at day 21.6,7
RAT ROTATOR CUFF TENDON HEALING
We also examined NOS expression in an acute rotator cuff tendon model in the rat by creating a defect in the supraspinatus tendon with a 3 mm diameter biopsy punch. In this model, all three NOS isoforms as determined by competitive reverse transcriptase-PCR were expressed. The expression profile was slightly different from that of healing rat Achilles tendon, with bNOS expression maximum on day 4, eNOS on day 7 and iNOS on day 7.8
RAT ROTATOR CUFF TENDON OVERUSE MODEL
In an exercise overuse model of tendon degeneration in the rat,9,10 we found that iNOS, eNOS and bNOS mRNAs were overexpressed in the supraspinatus tendon of rats subjected to treadmill running at 14 days.11 These results suggest that NOS activity is induced as a result of tendon injury in this model, and/or that expression of NOS is a part of supraspinatus tendinopathy.
HUMAN ROTATOR CUFF TENDON INJURY
During surgical repair of the rotator cuff in humans, the edges of the torn tendon are excised and discarded. We have evaluated these samples and found that NOS enzyme activity was detectable in 7 of 10 human rotator cuff tendon samples. mRNA expression was demonstrated for iNOS and eNOS isoforms in each sample examined, whereas bNOS mRNA was detectable in 3 of 8 samples.12 These results indicate that a similar phenomenon of NOS upregulation following injury occurs in humans as in rats.
WHERE DOES NO COME FROM?
In the rat Achilles tendon model, the first isoform to appear was iNOS, followed by eNOS and then bNOS. As one would expect, iNOS was expressed in macrophage-like cells and eNOS was found in endothelial cells.6 Interestingly, all three isoforms were expressed in fibroblast-like cells, again in a temporal fashion, with iNOS being expressed first (days 4–7), followed by eNOS (days 4–14) and bNOS (days 14–21).7
IS NOS EXPRESSION IMPORTANT TO TENDON HEALING?
We fed rats a competitive NOS inhibitor (Nώ-nitro-l-arginine methyl ester) and found that rats taking this inhibitor had significantly reduced healing of their Achilles tendons compared with rats drinking its inactive enantiomer (Nώ-nitro-d-arginine methyl ester). There was a 50% reduction in cross-sectional area of the Achilles tendon at day 7, and a corresponding 24% reduction in the failure load of the rat Achilles tendon constructs.5 We have also performed experiments on iNOS knockout mice and concluded that iNOS alone is not responsible for the beneficial effects of NO on tendon healing.13
WHAT ROLES DOES NO PLAY IN TENDON HEALING?
The experiments on animals using NOS inhibitors show that NO is important for the volume of tissue synthesised during tendon healing (fig 1). NO is likely to be important in a number of processes, including local blood flow and host defence. Work in our laboratory has identified NO to be important in collagen synthesis.14 Cultured human rotator cuff tendon cells, when exposed to exogenous NO in the form of S-nitro-N-acetyl penicillamine and when transfected with the iNOS gene via an adenovirus vector, incorporated more collagenase-sensitive 3H-proline in their matrix. This increased collagen synthesis was inhibited by an NOS inhibitor.
Schematic diagram of the overall effects of the addition and inhibition of nitric oxide (NO) on tendon healing.
We used microarray analysis to elucidate global gene expression after transfection with iNOS in tenocytes isolated from the injured rotator cuff tendons of humans. The expression of a wide range of genes was affected by NO, with many activated genes having known roles in healing. Of particular significance was that NOS overexpression stimulated the transcription and translation of a range of extracellular matrix genes important to the structure of connective tissues such as tendons, including collagen type I α1, collagen type III α1, collagen IV α5, biglycan, decorin, laminin and matrix metalloproteinase 10. These genes were also shown to respond to stimulation by the NO donor S-nitroso-N-acetyl-penicillamine in a dose-dependent manner.15 We also showed that varying levels of NO significantly affect cellular adhesion in tenocytes, a critical process during tendon repair.15
In our rat model, we delivered NO via flurbiprofen, a non-specific cyclo-oxygenase inhibitor and via NO-paracetamol. In both experiments, the addition of NO had a protective or beneficial effect on collagen organisation, tendon healing failure load and stress (load/area).16,17 These rat results are consistent with cell culture findings for human tendon cells where NO enhanced collagen synthesis, and with the results from the clinical trials described below.
CLINICAL TRIALS
To determine whether additional NO might enhance tendon healing in humans, we conducted three randomised double-blind clinical trials. These trials involved the application of a commercially available NO delivery system (glyceryl trinitrate (GTN) patches) and a placebo version of the same patch. These patches were applied over the area of tenderness for three conditions: tennis elbow, Achilles tendinosis and supraspinatus tendinosis (fig 2). A total of 53–86 patients were randomised to two groups in each of the trials. In each trial, the active group received continuous topical NO donation (1.25 mg/24 h GTN) and the placebo group received the identical patch without GTN. The GTN patches were applied to the area of maximal tenderness once a day. Both the patients and the clinical examiner were blinded as to which group the patients were in. In each trial, the patients also received education and exercises—that is, the GTN treatment was on top of “best practice”. The results of these trials can be found in Paoloni et al.18–20
Application of a glyceryl trinitrate (GTN) patch to the shoulder for supraspinatus tendinosis.
Dr Eugene Heyman completed an independent reanalysis of the data from the three studies. In this analysis any missing data, whenever it occurred, were filled in by carrying forward the last value. Each analysis was adjusted for the baseline value. For categorical data with responses of 0–4, the baseline value was treated as a block—that is, all the patients with a baseline value of 0 were grouped together, those with a baseline of 1 were grouped together and so on. The mean results were then compared between treatment groups within each baseline score and then the treatment differences were averaged while using the total number of patients within the baseline category to weight the results. Patients within the same baseline score were treated the same, but the treatment differences were weighted by the total number of patients within that baseline score. For continuous data (eg, total work), the change from baseline was used and then included as a covariate to account for any baseline differences and to account for the relationship between the baseline and the change from baseline. When patients with bilateral data were identified, the data were analysed in three ways. First, the results from the two sides were averaged, then, all bilateral patients were excluded. Finally, each group was treated separately. Tables 1–4 presents the results.
Genes upregulated or downregulated in cultured tendon cells following exposure to nitric oxide15
What this study adds
-
This study, involving three clinical trials, showed that delivering nitric oxide via a patch enhances clinical recovery of tendinopathy in humans.
-
The enhancement is manifested by a reduction in pain, an increase in range of motion and an increase in strength.
What is already known on this topic
-
Nitric oxide (NO) is important to tendon healing.
-
Nitric oxide synthase (NOS) activity is upregulated in tendinopathy.
-
In animal models where NOS activity is inhibited, tendon healing is reduced.
-
When additional NO is added, tendon healing is enhanced.
TENNIS ELBOW
The NO group had less tenderness and could perform more work and had greater peak power on the Orthopaedic Research Institute-Ankle Strength Testing System testing (table 2). The changes were most apparent at week 24. In all, 81% of patients receiving GTN patches were asymptomatic in activities of daily living at 6 months compared with 60% of patients with tendon rehabilitation alone (p = 0.005 with χ2 analysis).19
Effects of glyceryl trinitrate 1.25 mg/day through transdermal patch and rehabilitation versus rehabilitation alone on tennis elbow (adapted from Paoloni et al19)
ACHILLES TENDONITIS
The NO (GTN) group performed significantly better on hop testing and could generate more peak force at week 24 (table 3). In all, 78% of patients receiving GTN patches were asymptomatic for activities of daily living at 6 months compared with 49% of patients with tendon rehabilitation alone (p = 0.001 with χ2 analysis). The mean effect size for all outcome measures was 14%.18
Effects of glyceryl trinitrate 1.25 mg/day through transdermal patch and rehabilitation versus rehabilitation alone in patients with Achilles tendonitis (adapted from Paoloni et al18)
SUPRASPINATUS TENDINOPATHY
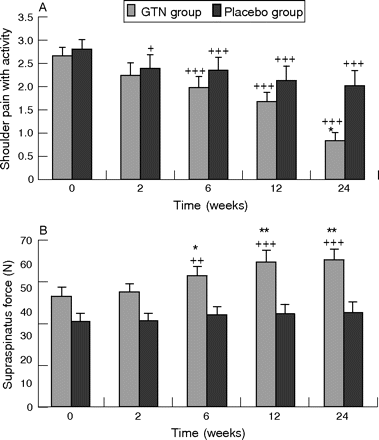
This trial produced the most significant effects. The NO group had significantly reduced shoulder pain with activity and at night, improved range of motion in abduction, forward flexion and external rotation, and improved power in abduction, external rotation, subscapularis and supraspinatus (table 4).20 The changes in supraspinatus power were the most dramatic, and were significant at 6 weeks (table 4, fig 3). In all, 46% of patients receiving GTN patches were asymptomatic for activities of daily living at 6 months compared with 24% of patients with tendon rehabilitation alone (p = 0.007). The mean effect size of GTN treatment for all outcome measures was 26%.20
Effects of glyceryl trinitrate 1.25 mg/day through transdermal patch and rehabilitation versus rehabilitation alone in patients with supraspinatus tendinopathy (adapted from Paoloni et al20)
Supraspinatus tendinosis. Effects of glyceryl trinitrate (GTN, n = 28) 1.25 mg/d via transdermal patch versus rehabilitation alone (placebo, n = 29) on shoulder pain with activities (A) and on dynamometer-measured supraspinatus force (B); a between-groups comparison of means and the standard error of the mean. Significant differences: *p<0.05, **p<0.01. ++p<0.01, +++p<0.001 compared with baseline using student’s paired t tests. (Adapted from Paoloni et al20).
DISCUSSION
NO is important to tendon healing. All three isoforms of NOS, the enzyme that produces NO, are expressed by fibroblasts during tendon healing. Our data in animal studies, cell culture and clinical trials support the hypothesis that NO enhances extracellular matrix synthesis and results in injured tendons having better material and mechanical properties—that is, the healing tendons are stronger on a per unit area basis than those not exposed to additional NO. The clinical trials show that delivering NO via a patch enhances the clinical recovery of tendinopathy, which is manifested by a reduction in pain, an increase in range of motion and an increase in strength.
Acknowledgments
I thank the following for their invaluable contributions: Tom Best, Allison Collins, Ed Lilly, Anthony Seaber, Richard Goldner, Kim Hayes, Judie Walton, Zoltan Szomor, Jian-Hao Lin, Min Wang, Ai Wei, Wei Zhu, Ashish Diwan, Danny Jang, Xian-Hao Deng, Jo Hannafin, Helen Davies, Marty Dolan, Dedee Murrell, Richard Appleyard, Janice Nelson, Fiona Bonar, Gong-Yao Tang, Jun Yuan, Piero Del Soldato, Wei Zhu, Wei Xia, Yao Wang, Denis King, James Urbaniak, Martin Francis, Robert Duthie, Ed Puzas, Charles Turner, Chris Evans, Bruce Caterson, Csaba Szabo, Laura Manfield, Justin Paoloni, Tim Molloy, Eugene Heyman, Robert Ang, National Institutes of Health Grant AR42729 (GACM), the Soft Tissue Research Fund, Piedmont Foundation, Orthopaedic Research and Education Foundation (OREF), St George Private Hospital/Health Care of Australia and St George Hospital/South East Health, and NiCOX Corporation and Cure Therapeutics.
REFERENCES
Footnotes
-
Published Online First 8 February 2007
-
Competing interests: None declared.