Article Text
Abstract
Participation in sports activity and regular physical training is associated with physiological structural and electrical changes in the heart (athlete’s heart) that enable sustained increases in cardiac output for prolonged periods. Cardiovascular remodelling in the conditioned athlete is often associated with ECG changes. In rare cases, abnormalities of an athlete’s ECG may reflect an underlying heart disease which puts the athlete at risk of arrhythmic cardiac arrest during sport. It is mandatory that ECG abnormalities resulting from intensive physical training and those of a potential cardiac pathology are properly defined. This article provides a modern approach to interpreting 12-lead ECGs of athletes based on recently published new findings. The main objective is to distinguish between physiological adaptive ECG changes and pathological ECG abnormalities. The most important aims are to prevent physiological changes in the athlete being erroneously attributed to heart disease, or signs of life-threatening cardiovascular conditions being dismissed as a normal variant of athlete’s heart. As pathological ECG abnormalities not only cause alarm but also require action with additional testing to exclude (or confirm) the suspicion of a lethal cardiovascular disorder, appropriate interpretation of an athlete’s ECG will prevent unnecessary distress and also result in considerable cost saving in the context of a population-based preparticipation screening programme.
Statistics from Altmetric.com
ECG changes in athletes are common and usually reflect structural and electrical remodelling of the heart as an adaptation to regular physical training (athlete’s heart).1 2 3 In rare cases, abnormalities of an athlete’s ECG may be an expression of an underlying heart disease putting the athlete at risk of sudden cardiac death (SCD) during sport.4 5 6 It is imperative that ECG abnormalities resulting from intensive physical training and those potentially associated with an increased cardiovascular risk are properly defined.7 8 9
This article provides a modern approach to interpreting 12-lead ECGs in athletes based on recently published new findings. The main objective is to differentiate between physiological adaptive ECG changes and pathological ECG abnormalities. The most important aims are to prevent physiological changes in the athlete being erroneously attributed to heart disease, or signs of life-threatening cardiovascular conditions being dismissed as normal variants of athlete’s heart. As pathological ECG abnormalities not only cause alarm but also require action with additional testing to exclude (or confirm) the suspicion of a lethal cardiovascular disorder, appropriate interpretation of an athlete’s ECG will prevent unnecessary distress and also result in considerable cost savings in the context of a population-based preparticipation screening programme.9
Athletes’ ECG abnormalities can be divided into two groups: common and training-related; uncommon and training-unrelated. This classification is based on prevalence, relation to exercise training, association with an increased cardiovascular risk, and need for further clinical investigation to confirm (or exclude) an underlying cardiovascular disease (table 1).
Classification of abnormalities of the athlete’s ECG
Trained athletes commonly (up to 80%) show ECG changes such as sinus bradycardia, first-degree atrioventricular (AV) block and early repolarisation, which result from physiological adaptation of the cardiac autonomic nervous system to athletic conditioning, such as increased vagal tone and/or withdrawal of sympathetic activity.10 Moreover, the ECGs of trained athletes often exhibit pure voltage criteria (ie, based only on QRS amplitude measurements) for left ventricular (LV) hypertrophy that reflect physiological LV remodelling with increased LV wall thickness and chamber size.
These ECG changes should be clearly separated from uncommon (<5%) and training-unrelated ECG patterns such as ST-T repolarisation abnormalities, pathological Q waves, intraventricular conduction defects, ventricular pre-excitation, long and short QT interval and Brugada-like repolarisation changes, which may be the expression of cardiovascular disorders, notably inherited cardiomyopathies or cardiac ion channel diseases, that may predispose to the risk of SCD.9 11
This classification of ECG abnormalities has important implications for the athlete’s cardiovascular management, including clinical diagnosis and risk stratification. Common ECG changes due to cardiac adaptation to physical exertion should not cause alarm, and the athlete should be allowed to participate in competitive sports without additional evaluation. Hence, further diagnostic work-up is only needed for the subset of athletes with uncommon and sports-unrelated ECG changes, which potentially reflect an underlying heart disease with an increased risk of SCD (group 2). This distinction of physiological from pathological ECG abnormalities provides favourable consequences for diagnostic accuracy and cost savings.
Common and training-related ECG changes
Training-related ECG abnormalities should be evaluated in light of the athlete’s gender, race, level of fitness, and type of sport.11 12 13 14 15 16 Physiological ECG abnormalities are more prevalent and significant in male athletes, athletes of African decent, and highly trained endurance athletes than in other athletic subgroups.11 15 16 This probably reflects the effect of genetic/ethnic predisposing factors, which account for a more prominent cardiovascular remodelling, either structural or neuroautonomic, in response to physical training and competition.13 14 Level and duration of training or competition, aerobic capacity and type of sports activity play an important role as well. Participation in sports that require high endurance, such as cycling, cross-country skiing and rowing/canoeing, has been shown to be significantly associated with a higher rate, and greater extent, of physiological ECG changes such as sinus bradycardia and increase in QRS voltages compared with participation in sports that require more strength and speed and less endurance.11 This seems to be related to the large cardiac output acquired during endurance training, resulting in considerable cardiac remodelling including increases in LV cardiac dimension and wall thickness.17
Sinus bradycardia/arrhythmia
Resting sinus bradycardia, as defined by a heart rate <60 beats/min, is almost universal in athletes, depending on the type of sport and the level of training/competition.18 19 20 Escape junctional beats or rhythm may be recorded in athletes with more severe bradycardia and result in functional AV dissociation. Sinus arrhythmia is also reported with widely varying frequency, from approximately 15% to 70%.18 21 Sinus bradycardia/arrhythmia disappear during exercise, suggesting that high vagal tone causes slowing of the sinus atrial node.
Work-up
Bradycardia is the result of a physiological adaptive change of the autonomic nervous system and reflects the level of athletic conditioning. Only profound sinus bradycardia and/or marked sinus arrhythmia (<30 beats/min) need to be distinguished from sick sinus syndrome. A sinus atrial node dysfunction can be reasonably excluded by demonstrating that: (1) the decrease in heart rate is appropriate for the level of training and type of sports; (2) symptoms, such as dizziness or syncope, are absent; (3) heart rate normalises during exercise, sympathetic manoeuvres or drugs, with preservation of maximal heart rate; and (4) bradycardia reverses with training reduction or discontinuation.
AV block
First-degree AV block and Mobitz type I (Wenkebach) second-degree AV block are commonly seen in trained athletes, being present in ∼35% and 10% of athletes’ ECGs, respectively.21 22 23 As with sinus bradycardia, AV conduction slowing and block are mediated by increased parasympathetic tone and/or decreased resting sympathetic tone.
Work-up
Resolution of (asymptomatic) first-degree or second-degree AV block with hyperventilation or exercise confirms its functional origin, and excludes any pathological significance. Type II second-degree (Mobitz type II) and third-degree AV block should prompt exclusion of associated symptoms or underlying structural heart disease.
Isolated increase in QRS voltages
Intensive athletic conditioning is associated with morphological cardiac changes, including increased cavity dimensions, wall thickness and ventricular mass, which are reflected on the 12-lead ECG.1 2 3 The ECG patterns of physiological LV hypertrophy in trained athletes usually manifests as an isolated increase in QRS amplitude, with normal QRS axis, normal atrial and ventricular activation patterns, and normal ST-segment–T-wave repolarisation.11 20 21 24 25 26 Several studies have reported a high incidence (up to 80%) of athletes’ ECGs that fulfil electrocardiographic LV hypertrophy if the criteria of Sokolow and Lyon are used (S wave in V1 + R wave in V5>35 mm).11 23 27 Non-voltage ECG criteria for LV hypertrophy such as atrial enlargement, left axis deviation, a “strain” pattern of repolarisation and delayed intrinsicoid deflection are usually not seen in athletes. These ECG abnormalities infer an underlying pathological LV hypertrophy, as a result of hypertrophic cardiomyopathy (HCM), aortic valve disease or hypertensive heart disease.
Work-up
Athletes showing an isolated increase in QRS voltage on their 12-lead ECG do not require systematic echocardiographic evaluation, unless they have other non-voltage ECG criteria suggesting pathological LV hypertrophy, relevant symptoms or a positive family history of cardiovascular diseases and/or premature SCD.
Incomplete right bundle branch block (RBBB)
The prevalence of incomplete RBBB (QRS duration <120 ms) has been estimated to range from 35% to 50% in athletes compared with 10% in young, healthy controls.11 25 28 29 30 31 The ECG pattern is more often noted in athletes engaged in endurance sports, with a striking male preponderance. It has been suggested that the right ventricular (RV) conduction delay is not within the His-Purkinje system, but is caused by the enlarged RV cavity size/increased cardiac muscle mass and the resultant conduction delay.28
Work-up
Incomplete RBBB does not require further tests in the presence of a negative family/personal history and physical examination. Because incomplete RBBB is a typical ECG finding in patients with an atrial septal defect of the “ostium secundum” type, particular attention should be paid to exclude related symptoms and a fixed split of the second tone by accurate cardiac auscultation.
Typical features of incomplete RBBB are uncommonly observed in patients with arrhythmogenic RV cardiomyopathy/dysplasia (ARVC/D).32 An underlying ARVC/D should be suspected when the pattern of incomplete RBBB is associated with disproportionate extent of T-wave inversion (beyond V2 to include midprecordial V3 and V4 leads) or in the presence of premature ventricular beats with a left bundle branch block (LBBB) morphology.
In some cases, incomplete RBBB should be differentiated from a Brugada ECG. The ECG pattern of the ion channel disorder, Brugada syndrome, is characterised by a slow, positive deflection at the R–ST junction (“J wave”), which is most evident in leads V1 and V2, with minimal or no reciprocal changes in other leads33 (fig 1). Unlike the R′ wave seen in RBBB, the J wave suggestive of Brugada syndrome does not indicate a RV delayed activation, but, rather, early repolarisation with J-point elevation and a high take-off ST segment. The down-sloping ST segment is followed by a negative (“coved” type) or a positive (“saddle-back” type) T wave. In typical RBBB, the R′ wave recorded in V1 and V2 is distinctively associated with reciprocal S waves in L1 and V6, and the right precordial leads do not show any elevation of the ST segment.34 Differential diagnosis may require in selected cases a drug challenge with sodium channel blockers (fig 1B).
(A) Borderline Brugada ECG pattern mimicking incomplete right bundle branch block (RBBB). Unlike the R wave of RBBB, the J wave (arrows) of the Brugada ECG is confined to right precordial leads (V1 and V2) without reciprocal S wave (of comparable voltage and duration) in the leads L1 and V6 (arrowhead). (B) In this case, a definitive diagnosis from the Brugada ECG was achieved by a drug challenge with sodium channel blockers, which unmasked a diagnostic “coved type” (arrows) pattern (V1 and V2).
Early repolarisation
Early repolarisation has traditionally been regarded as an idiopathic and benign ECG phenomenon, with an estimated prevalence in healthy young people ranging between 1% and 2%, and a clear male preponderance.35 36 37 38 The early repolarisation ECG pattern is the rule rather than the exception among highly trained athletes, in whom it is observed in 50–80% of resting ECGs.39 40 41 The most notable ECG feature is the elevation of the QRS–ST junction (J point) of at least 0.1 mV from baseline, often associated with notching or slurring of the terminal QRS complex. Early repolarisation may vary on location, morphology and degree.37 38 It is most often localised in precordial leads, with the greatest ST-segment elevation in mid-to-lateral leads (V3–V4). Maximal ST-segment displacement may also occur more laterally (leads V5, V6, L1 and aVL), inferiorly (L2, L3 and aVF) or anteriorly (leads V2–V3).38 41 42 The most common morphological pattern seen in the Caucasian population is characterised by an elevated ST segment with an upward concavity, ending in a positive (“peaked and tall”) T wave (fig 2A). In athletes of African–Caribbean descent, a common pattern consists of an elevated ST segment with an upward convexity, followed by a negative T wave (fig 2B) in V2–V4. The latter pattern, which is due to the “domed” morphology of the elevated ST segment, may raise the problem of a differential diagnosis with the Brugada ECG (see under “Brugada-like ECG abnormalities” below).41 42
Different patterns of precordial early repolarisation in two healthy athletes. (A) ST-segment elevation with upward concavity (arrows), followed by a positive T wave (arrowheads). (B) ST-segment elevation with upward convexity (arrows), followed by a negative T wave (arrowheads).
The magnitude of ST-segment elevation is characteristically modulated by autonomic influences, heart rate changes and drugs; this explains the dynamic nature of the ECG abnormalities and a waxing and waning of the ST-T segment over time.37 Slowing of the heart rate exaggerates ST-segment elevation, whereas sinus tachycardia occurring during exercise or after isoproterenol administration reduces and often eliminates early repolarisation changes.
Recently a significantly increased prevalence of the ECG pattern of early repolarisation in the inferior and/or lateral leads with terminal QRS slurring has been reported among patients with a history of idiopathic ventricular fibrillation.43 The study was a retrospective analysis of a very selected patient cohort with episodes of short coupled rapid/polymorphic ventricular tachycardia or ventricular fibrillation leading to syncope or cardiac arrest. The available data do not provide evidence that, in the general population of asymptomatic young people or athletes, this ECG pattern is predictive of an increased risk of malignant ventricular arrhythmias.
Work-up
Early repolarisation is a physiological and benign ECG pattern in the general population of young people and athletes, and does not require further clinical evaluation. In trained athletes, right precordial ST-T changes due to early repolarisation show typical features that may allow differentiation from ARVC/D (fig 3) or Brugada syndrome (fig 4).41 42 44 45 In rare cases, athletes may require pharmacological testing with sodium channel-blocking agents, electrophysiological study or cardiac imaging study to achieve a conclusive diagnosis.
(A) Early repolarisation pattern in a healthy black athlete characterised by right precordial T-wave inversion (arrowhead) preceded by ST-segment elevation (arrow). (B) Right precordial T-wave inversion in a patient with arrhythmogenic RV cardiomyopathy/dysplasia (ARVC/D). Note that, unlike early repolarisation, in ARVC/D the right precordial leads do not show any elevation of the ST segment.
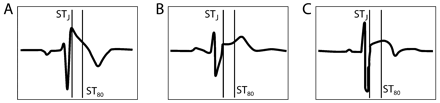
Differential diagnosis between representative right precordial ECG patterns from (A) a patient with Brugada syndrome and (B,C) two trained athletes. Vertical lines mark the J point (STJ) and the point 80 ms after the J point (ST80) where the amplitudes of ST segment elevation are calculated. “Coved” type ST-segment elevation in the patient with Brugada syndrome is characterised by a “down-sloping” elevated ST segment with a STJ/ST80 ratio of 1.9. Right precordial early repolarisation patterns in both athletes show an “up-sloping” ST-segment elevation with STJ/ST80 ratio <1: 0.7 for the “concave” toward the top (B) and 0.68 for the “convex” toward the top (C) ST segment elevation. Modified from Corrado et al.42
In athletes presenting with syncope or cardiac arrest, which remains unexplained after a detailed clinical work-up aimed to exclude cardiac causes and neuromediated mechanisms, an ECG pattern of early repolarisation in inferior and/or lateral leads, with a prominent terminal QRS slurring, should raise the suspicion of an underlying idiopathic ventricular fibrillation.43
Uncommon and training-unrelated ECG changes
Most cardiovascular conditions responsible for SCD in young competitive athletes are clinically silent and unlikely to be suspected or diagnosed on the basis of spontaneous symptoms.4 5 6 The 25-year Italian screening experience has shown that 12-lead ECG, in addition to history and physical examination, has substantial value for identifying asymptomatic athletes who have potentially lethal heart disorders, and actually saves lives.46 47 48 49 50 51 52 ECG-detectable cardiovascular diseases include: cardiomyopathies, such as HCM, ARVC/D and dilated cardiomyopathy; aortic valve stenosis; cardiac ion-channel diseases such as long QT syndrome (LQTS), Brugada syndrome, short QT syndrome and Lenegre disease; and Wolff–Parkinson–White syndrome. On the basis of published series from the USA and Italy, overall these conditions account for approximately two-thirds of SCD in young competitive athletes. ECG abnormalities associated with these cardiovascular diseases include repolarisation abnormalities such as inverted T waves and ST-segment depression, pathological Q waves, intraventricular conduction defects, ventricular pre-excitation, long and short QT interval, and Brugada-like repolarisation changes (table 1).
Unlike the ECG changes characteristic of athlete’s heart, such potentially risky ECG abnormalities are relatively uncommon (<5%) and training-unrelated. Further diagnostic work-up is mandatory for those athletes who exhibit such ECG changes in order to confirm (or exclude) an underlying cardiovascular disease.
Non-voltage criteria for LV hypertrophy
HCM is one of the leading causes of SCD in apparently healthy competitive athletes age <35 years.53 This condition is often in the differential diagnosis with adaptive changes of athlete’s heart. ECG has the potential to accurately distinguish between physiological and pathological hypertrophy, given that ECG abnormalities of HCM overlap marginally with training-related ECG changes. An isolated QRS voltage criterion for LV hypertrophy (Sokolow–Lyon or Cornell criteria) is a very unusual pattern (∼1.9%) in patients with HCM in whom pathological LV hypertrophy is characteristically associated with one or more additional non-voltage criteria such as left atrial enlargement, left axis deviation, delayed intrinsicoid deflection, ST-segment and T-wave abnormalities, and pathological Q waves.54 55 56
Work-up
Regardless of family and personal history, athletes with non-voltage criteria for LV hypertrophy require an echocardiographic evaluation to exclude underlying structural heart disease and pathological LV hypertrophy, including HCM.
ST-segment depression
Although ST-segment elevation due to early repolarisation is a common finding in the basal ECG of trained athletes, resting ST-segment depression is rarely observed. In the literature, ST-segment depression is usually lumped together with T-wave inversion, making the real incidence of isolated ST-segment depression unknown.
Work-up
Demonstration of ST-segment depression on resting ECG, either isolated or associated with T-wave inversion, should prompt further investigations to exclude heart disease.
Right atrial enlargement and RV hypertrophy
ECG criteria for right atrial enlargement and/or RV hypertrophy are uncommon findings in athletes. Pelliccia et al11 reported a prevalence of 0.08% for right atrial enlargement and 0.6% for a right axis deviation (>110°) among a large cohort of highly conditioned athletes. The Sokolow–Lyon voltage criterion for RV hypertrophy (R−V1 + S−V5>10.5 mm) was met in one of 172 (0.6%) professional soccer players.57 A higher prevalence of the Sokolow–Lyon voltage criterion for RV hypertrophy was reported by Sharma et al27 among junior elite athletes (12%), although there was no difference from controls (10%). A significant proportion of athletes and non-athletes in this study was younger than 16 years: in this age group a voltage criterion for RV hypertrophy is more common.
Work-up
The ECG pattern of right atrial enlargement and/or RV hypertrophy should not be simply interpreted as a manifestation of exercise-induced cardiac remodelling. The presence of either congenital or acquired heart diseases associated with an increased right atrial size and/or pathological RV dilatation/hypertrophy should be excluded by an appropriate imaging study.
T-wave inversion
Recent studies on large athletic populations have disproved the traditional idea that T-wave inversions are common and training-related ECG changes in the athlete. Pelliccia et al11 reported a 2.7% prevalence of T-wave inversion in 1005 highly trained athletes and 2.3% in a large population of 32 652 young amateur athletes. However, Sharma et al27 reported that the prevalence of T-wave inversion is similar among elite athletes and sedentary controls (4.4% vs 4.0%, respectively). The presence of T-wave inversion ⩾2 mm in ⩾2 adjacent leads in an athlete is a non-specific but warning ECG sign of a potential cardiovascular disease with the risk of SCD during sport. T-wave inversion in inferior (L2, L3, aVF) and/or lateral (L1, aVL, V5–V6) leads must raise the suspicion of ischaemic heart disease, cardiomyopathy, aortic valve disease, systemic hypertension and LV non-compaction. The postpubertal persistence of T-wave inversion beyond V1 may reflect an underlying congenital heart disease leading to a RV volume or pressure overload state, an ARVC/D, or, uncommonly, an inherited sodium/potassium channel disease. A recent study showed that T-wave inversion beyond V1 is seen in postpubertal athletes less commonly than previously thought (1.4%), but deserves special consideration because it may reflect underlying ARVC/D.58
T-wave inversion in young and apparently healthy athletes may represent the initial phenotypic expression of an underlying cardiomyopathy, before the development of morphological changes detectable on cardiac imaging. Thus, failure to detect structural abnormalities on imaging does not exclude T-wave inversion due to disease of the heart muscle, as this may only become evident many years later and may ultimately be associated with an adverse outcome.59 60
Work-up
T-wave inversion ⩾2 mm in ⩾2 adjacent leads is rarely observed on the ECG of healthy athletes, whereas it is a common finding in patients with cardiomyopathy. Inverted T waves may represent the only sign of an inherited heart muscle disease even in the absence of any other features or before structural changes in the heart can be detected. Hence, the perspective that T-wave inversion is due to cardiovascular adaptation to physical exercise should only be accepted once inherited forms of cardiovascular disease have been definitively excluded by a comprehensive clinical work-up, including screening of family members/first-degree relatives, and molecular genetic testing when available. In this regard, athletes with postpubertal persistence of T-wave inversion beyond V1 require further clinical and echocardiographic evaluation to exclude an underlying cardiomyopathy such as ARVC/D or HCM. The recent observation that T-wave inversion may identify athletes at risk of subsequent development of structural heart disease underscores the importance of continued clinical surveillance and follow-up by serial ECG and echocardiography evaluations of trained athletes with T-wave repolarisation abnormalities, even in the absence of clinically demonstrable heart disease.
The significance of minor T-wave changes such as flat and/or minimally inverted (<2 mm) T waves in ⩾2 leads (mostly inferior and/or lateral) is unclear. These changes usually revert to normal with exercise and are considered a benign ECG phenomenon resulting from increased vagal tone. Like deep inverted T waves, however, such minor T-wave abnormalities are uncommonly encountered in the athlete heart (<0.5%),27 but are common in cardiomyopathy. This indicates that they may have a pathological basis and should be cautiously investigated and followed-up over time before they are definitively ascribed to physiological neuroautonomic remodelling.
Intraventricular conduction abnormalities
Complete RBBB and LBBB (QRS duration ⩾120 ms) and left anterior and posterior hemiblocks are not commonly seen in athletes (<2% of athletes’ ECGs) and represent a potential marker of malignant cardiovascular diseases.61 62 63 64 Demonstration of such intraventricular conduction abnormalities should lead to a complete cardiological work-up including exercise testing, 24 h Holter monitoring and imaging techniques for evaluation of underlying pathological causes. An ECG should be obtained in the siblings of a young athlete with an ECG pattern of bifascicular block (ie, LBBB, RBBB and left anterior hemiblock, or RBBB and left posterior hemiblock) to exclude a genetically determined progressive cardiac conduction disease (Lènegre disease).65
Non-specific intraventricular conduction defects
A prolonged QRS (>110 ms) not satisfying the criteria for either LBBB or RBBB is referred to as non-specific intraventricular conduction defect.34 Because the conduction delay occurs in the ventricular myocardium rather than in the specialised conduction system, this conduction defect is a special ECG indicator of a possible heart muscle disease and requires accurate cardiovascular investigation. For instance, localised prolongation of the QRS complex (>110 ms) in the leads exploring the right ventricle (V1–V3), often associated with an “epsilon wave” (ie, a terminal notch in the QRS complex) and/or delayed S-wave upstroke, is considered to be an ECG marker for ARVC/D (fig 5).
ECG recording of a patient with arrhythmogenic right ventricle (RV) cardiomyopathy/dysplasia showing a non-specific RV conduction defect, which is characterised by an increase in QRS duration (115 ms) in the right precordial leads, associated with an epsilon wave (arrow) in V1 (ie, a low-amplitude, low-frequency wave occurring after the end of the QRS) and a prolonged S-wave upstroke in V1 and V2 (arrowhead).
Ventricular pre-excitation (Wolff–Parkinson–White)
The prevalence of ventricular pre-excitation in the general population varies from 0.1% to 0.3% and does not differ in athletic populations. Sports activity in the presence of overt pre-excitation may expose the athlete to an increased risk of SCD if the AV accessory pathway has the potential for fast antegrade conduction.66 67 Athletes with ventricular pre-excitation should be referred to a specialist for evaluation by electrophysiological study, either transoesophageal or intracardiac, for the inducibility of AV re-entrant tachycardia and the anterograde refractory period of the accessory pathway (shortest pre-excited RR interval at rest and during exercise or adrenergic drug stimulation), which influence eligibility for athletic competition, risk stratification and treatment, including catheter ablation.
Long and short QT interval
Demonstration of a QTc value (ie, QT interval corrected by heart rate using Bazett’s formula) ⩾500 ms, otherwise unexplained, is indicative of unequivocal LQTS, regardless of family history and symptoms. Borderline QTc prolongation <500 ms requires further evaluation to achieve a conclusive diagnosis.68 Twenty-four hour Holter monitoring may allow recording of more pronounced (diagnostic) QTc prolongation or associated ST-T morphological abnormalities over time, T-wave alternans and polymorphic ventricular tachycardia. Exercise testing may enhance diagnostic accuracy because shortening of the QT interval during effort is inadequate and/or repolarisation abnormalities become more prominent and recognisable after exercise (in the recovery phase) in patients with LQTS. The ECG response to exercise may vary according to LQTS genotype: the QTc prolongs in LQT1, remains unchanged in LQT2, and shortens excessively in LQT3 patients.69 Athletes with a clear-cut prolonged QTc interval should be referred to a cardiac specialist for definitive diagnosis and risk stratification of LQTS, including molecular screening of causative gene mutations.
A short QT syndrome should be considered in the presence of a QTc interval <340 ms and no evidence of secondary QT interval shortening.70 The identification of an abnormally short QT interval in an athlete should enable familial cascade screening and molecular genetic evaluation.
Brugada-like ECG abnormalities
Brugada syndrome should be considered in the presence of an early, high-take-off and down-sloping ST-segment elevation (J wave) of either the coved (negative T wave) or saddle-back (positive T wave) type in V1–V3 (fig 1), in the absence of other causes of right precordial ST-segment elevation, such as cardiomyopathy, myocarditis or antidepressive drugs.71 72 Analysis of the ST-T waveform usually permits differential diagnosis with right precordial early repolarisation seen in athlete’s heart. Athletes exhibit an up-sloping ST segment with a mean STJ/ST80 ratio ⩽1, whereas patients with Brugada syndrome show a down-sloping ST segment with a STJ/ST80 ratio >1 (fig 4). In very selected cases, a pharmacological test with sodium channel-blocking agents is required to achieve a definitive diagnosis. The athlete with a diagnosis of Brugada ECG should be referred to a cardiologist/electrophysiologist for risk stratification and familial clinicogenetic screening.
Conclusions
The future for prevention of sports-related SCD by a population-based ECG screening programme lies in continuing efforts aimed to further understand the scientific basis for ECG interpretation and to better define standards of ECG criteria for differentiation between athlete’s heart and true heart diseases, taking into account variations by gender, ethnicity and various types and levels of sports activity. Use of modern ECG criteria to distinguish physiological from pathological changes in trained athletes will result in improved accuracy and cost-effectiveness when screening athletes for cardiovascular diseases that predispose to SCD.
What is already known on this topic
The presumption that ECG is a poor screening tool for cardiovascular disorders in athletes is based on (1) the knowledge that ECG abnormalities occur often in trained athletes as a consequence of adaptive changes of the heart to sustained physical exercise, and (2) the misconception that most ECG abnormalities in athletes overlap with ECG findings of cardiovascular diseases that hold the risk of sudden death.
What this study adds
The long-term Italian experience with preparticipation ECG screening has disproved the old concept of the low cost-effectiveness of ECG testing. This article provides cardiologists and sports medicine physicians with a modern approach to the correct interpretation of 12-lead ECGs in athletes.
REFERENCES
Footnotes
Funding This work was supported by the Italian Society of Sports Cardiology (SIC Sport), the Italian Federation of Sports Medicine (FMSI) and the Fondazione Cariparo, Padova e Rovigo, Italy.
Competing interests None.
Provenance and peer review Commissioned; externally peer reviewed.