Article Text
Statistics from Altmetric.com
Background
Guidelines on physical activity or exercise and pregnancy encourage pregnant women to continue or adopt an active lifestyle during and following pregnancy.1–3 Two systematic reviews of pregnancy-related guidelines on physical activity found similarities between recommendations from different countries, but noted that the guidelines differed in focus.4 ,5 The guidelines provided variable guidance on prenatal exercise, or on how pregnant women might approach continuing or adopting sport activities.6 However, most guidelines did not include important topics such as prevalence and known risk factors for common pregnancy-related diseases and complaints, and the role of exercise in preventing and treating them.
Importantly, the focus of most previous guidelines has been on healthy pregnant women in the general population, in whom there is almost always a decline in physical activity during pregnancy.7 ,8 Indeed, a high proportion of pregnant women follow neither physical activity nor exercise guidelines,9 putting them at increased risk of obesity, gestational diabetes mellitus (GDM), and other pregnancy-related diseases and complaints.1
On the other hand, there are enthusiastic exercisers and elite athletes who often meet and exceed general exercise recommendations for pregnant women, but there are no exercise guidelines specifically for these women. Important questions for such women are unanswered in current guidelines: Which activities, exercises and sports can they perform, for how long and at what intensity, without risking their own health and the health of the fetus? How soon can they return to high-intensity training and competition after childbirth?
The IOC and most National Sports Federations encourage women to participate in all Olympic sport disciplines. The IOC promotes high-level performance, and it is also strongly committed to promoting lifelong health among athletes10—not just during their competitive sporting careers. With an increasing number of elite female athletes competing well into their thirties, many may wish to become pregnant, and some also want to continue to compete after childbirth. With this background, the IOC assembled an international expert committee to review the literature on physical activity and exercise (1) during pregnancy and (2) after childbirth, using rigorous systematic review and search criteria.11 For efficiency, where sex is not specified, the reader should assume that this manuscript about pregnancy and childbirth refers to females (ie, ‘the elite athlete who wishes to train at altitude’ is used in preference to ‘the elite female athlete…’).
Aims
The September 2015 IOC meeting of 16 experts in Lausanne had three aims. They were to:
Summarise common conditions, illnesses and complaints that may interfere with strenuous exercise and competition, during pregnancy and after childbirth;
Provide recommendations for exercise training during pregnancy and after childbirth, for high-level regular exercisers and elite athletes; and
Identify major gaps in the literature that limit the confidence with which recommendations can be made.
Methods
For each section of the document, a search strategy was performed using search terms such as ‘pregnancy’ OR ‘pregnant’ OR ‘postpartum’ AND ‘exercise’ OR ‘physical activity’ OR ‘leisure activity’ OR ‘leisure’ OR ‘recreation’ OR ‘recreational activity’ or ‘physical fitness’ OR ‘occupational activity’ AND terms related to the condition under study (eg, ‘gestational diabetes’). Available databases were searched, with an emphasis on PubMed, EMBASE, Cochrane, PEDro, Web of Science and SPORTDiscus. In addition, existing guidelines with reference lists were scanned.
The review of each topic followed the general order: prevalence of the condition in the general pregnant or postpartum population, prevalence in high-level exercisers or elite athletes, risk factors in the general population and in relation to exercise and sport, and effect of preventive and treatment interventions. Level of evidence and grade of recommendations are according to the Cochrane handbook (table 1) for prevention and treatment interventions only.
Levels of quality of a body of evidence in the GRADE statement
Each member of the working group was assigned to be the lead author of one or more topics and 1–3 others were assigned to review each topic. A first full consensus draft was reviewed before and during the 3-day IOC meeting (27–29 September 2015), and a new version of each topic was submitted to the meeting chairs (KB and KMK) shortly after the meeting. Each topic leader made amendments before sending a new version for comments to the working group.
Preconception: applying current knowledge to the care of the elite athlete
The goal of preconception care is to optimise the woman's health and knowledge before planning, and conceiving a pregnancy and thus reduce the risk of adverse health effects for the woman, fetus or neonate. Because reproductive capacity spans almost four decades, optimising women's health before and between pregnancies is an ongoing process that requires access to, and the full participation of, all segments of the healthcare system.13
Preconception care aims to identify and modify biomedical, behavioural and social risks through preventive management intervention.14 The American Academy of Paediatricians and the American College of Obstetrics and Gynaecology classify this care into four categories:13 physical assessment, risk assessment, vaccinations and counselling. For the elite athlete, a number of particular areas should be highlighted and addressed.
Increasing age is associated with decreased fertility15 and increased rates of chromosomal abnormalities.16
To optimise fertility without in vitro fertilisation (IVF), one research group recommends that couples should start attempting conception at no later than 32 years of age if they are planning a one-child family, 27 years for a two-child family or 23 years for three children. If couples accept a 75% or lower chance of family completion, they can start 4–11 years later. If IVF is an option, couples can delay trying to conceive until the female partner is 35 years of age or younger for a one-child family, or until 31 years for two children, or 28 years for three children.17
This age period coincides with peak performance for many athletes, who may have impaired fertility related to relative energy deficiency in sport (RED-S),18–21 a consideration when making reproductive health recommendations to athletes. RED-S refers to impaired physiological function including, but not limited to, metabolic rate, menstrual function, bone health, protein synthesis, immunological health and cardiovascular health caused by relative energy deficiency.18 The long-term reproductive repercussions of RED-S for women are unknown and further research is required.18 Numerous features of RED-S have a negative impact on fertility. These include disordered eating and the associated hormonal imbalance. The reader interested in this topic is referred to the IOC Consensus Statement on RED-S.18–21
Eating disorders, pregnancy and athletes
There is consistent evidence that the frequency of eating disorders is higher among athletes (20–22%) than non-athletes (3–9%), with an especially high prevalence in weight-sensitive sports such as endurance (24%), aesthetic (42%) and sports with weight (30%) categories.22–25 Competitive athletes are constantly under the stress of improving their results and adapting to the sport-specific ‘ideal’. Important risk factors for severe eating disorders include pressure to lose weight, the early start of sport-specific training, overtraining, injuries, restriction of food intake and individual vulnerability.22 ,25 In addition, the sport environment and inappropriate coaching behaviour can exacerbate the issue.25 Those with a severe eating disorder are at risk for both medical complications and death, with a reported mortality rate between 4% and 10%.26
Pregnant athletes with an eating disorder, and their offspring, are at particular risk as they compete for limited nutritional resources. To our knowledge, there are no data on prevalence of eating disorders in pregnant athletes.
Complications associated with eating disorders
Pregnancy complications for women with anorexia nervosa include hyperemesis gravidarum, anaemia, spontaneous abortion, preterm birth (PTB), caesarean section, and postpartum depression.27–29 In addition, the neonate is at risk for low birth weight (LBW) and small head.30 ,31 Some studies have shown that these infants have developmental disturbances that persist throughout childhood and adulthood compared with children born to mothers without eating disorders.31 ,32 These children are also more prone to develop anxiety, depression and substance abuse.27 ,32
Pregnancy complications associated with bulimia nervosa are similar to those reported with anorexia nervosa. Additional complications include increased risk of vaginal bleeding, hypertension, fetal abnormalities, low Apgar scores, breech delivery and stillbirth.27 ,33
Management and treatment
If a pregnant athlete were to have symptoms of, or be considered at risk for, abnormal eating behaviour by medical staff, coaches or other off-field team members, the first step would be to refer appropriately (eg, to a nutritionist or psychiatrist, if appropriate). Close follow up by a team of nutrition, maternal-fetal medicine and psychiatric specialists is required to optimise maternal and fetal outcomes.27 ,34 In severe cases, management may require hospitalisation.
Conclusion
The pregnant athlete with a past or current eating disorder should be considered as being at higher risk of pregnancy complications and requires close monitoring by hypertension, miscarriage, difficult labour, premature delivery and intrauterine growth restriction. Hence, they should be monitored closely, including involvement of a multidisciplinary team, emphasising early recognition and treatment of symptoms, meal planning, training regimen adjustments, and evaluation for maternal or fetal consequences of malnutrition.
Pregnancy
Anatomical and physiological adaptations to pregnancy during each trimester
During pregnancy, there are major changes in the body's physiology and morphology. In this section, we highlight how regular or intense exercise influences the pregnancy-related changes. For a comprehensive overview of the physiology of normal pregnancy, unrelated to exercise and sport, we recommend references.35 ,36
Musculoskeletal adaptations to pregnancy
The expanding uterus displaces the centre of gravity, which results in the woman compensating to avoid falling forward. This may result in progressive lumbar lordosis and anterior rotation of the pelvis on the femur,37 though this finding is not uniformly observed.38 If there is an increase in lordosis, then an increase in anterior flexion of the cervical spine and abduction of the shoulders also takes place, which may interfere with performance in specific sports. Enlarged breasts also contribute to the shift in the centre of gravity.
During late pregnancy and the early postpartum period, most often there are significant decreases in both the length of the gait cycle and step length,39–43 and a significant increase in double support time.40 ,41 ,44 Additionally, single support time is reduced41 ,44 and step width increased leading to a wider stance.42 ,45
During pregnancy, the anterior tilt of the pelvis increases by approximately 5°,39 ,44 followed with an increase in hip flexion during stance phase,39 ,40 an increase of knee flexion during the terminal stance phase,41 a decrease of knee extension,40 and a decrease of ankle dorsiflexion and plantar flexion.39 ,40
Our search revealed no studies on biomechanical changes in regular exercisers or in elite athletes.
How muscle changes with pregnancy
There are few studies on the muscle morphology of pregnant women. Changes in hormone receptors and their regulators may promote changes in skeletal muscle fibre type (from oxidative to glycolytic).46
How balance changes with pregnancy
Postural balance is affected after the first trimester of pregnancy.47 Subsequently, falling is a common cause of injury in the general pregnant population, and pregnant women are 2–3 times more likely to be injured by falling than are non-pregnant women.47 ,48 Physical activity and exercise may mitigate this risk, but this has not been tested in the research setting.
Cardiorespiratory, metabolic and thermoregulatory adaptations to pregnancy
Hormones and growth factors are released into the maternal system in early pregnancy by the corpus luteum, placenta and developing embryo.49 These trigger a cascade of physiological events that regulate implantation and fetal–placental growth and development,50 leading to maternal, placental and fetal adaptations that occur in a normal low-risk pregnancy.
Cardiorespiratory adaptations to pregnancy
From about the fifth week of gestation, pregnancy induces rapid, progressive and substantial alterations to the cardiovascular system,51 which ensure blood supply to the fetus.52 Oestrogen-mediated remodelling reduces vascular tone, leading to a primary reduction in afterload and an increase in venous capacitance.51 This is reflected in increased resting cardiac output of about 50% over non-pregnant values.53
Remodelling of the heart increases the dimensions of the ventricular cavity54 without increasing wall thickness,55 increases aortic capacitance56 and reduces peripheral vascular resistance.57 There is a 15–20 bpm increase in resting HR over non-pregnant values.58 Stroke volume also increases by approximately 10% by the end of the first trimester.59 This manifests before significant enhancement in maternal blood volume,51 which may increase up to 50% above pre-pregnancy values by late pregnancy.60 ,61
There are also pregnancy-induced adaptations to the maternal respiratory system. For example, remodelling and expansion of the thoracic cage raises diaphragmatic mid-position;62 this decreases residual volume and expiratory reserve volume.63 One of the most substantial physiological pregnancy-induced changes, possibly designed to protect against fetal acidosis,64 is an increase in respiratory sensitivity to carbon dioxide (CO2) early in pregnancy. This increases tidal volume and minute ventilation, which reduces arterial carbon dioxide tension and increases arterial oxygen tension.65 These changes create a buffer that protects the fetus from any acute elevations in maternal carbon dioxide levels.66
Many pregnant women complain of respiratory discomfort (dyspnoea), especially in late pregnancy, both at rest and after exertion.67 Resting oxygen uptake (relative–mL/kg/min) reflects the increase in body mass during pregnancy, and thus declines slightly during each trimester.35 However, during submaximal steady-state exercise, pregnant women's perceptions of respiratory effort and dyspnoea seem to be reduced,68 because maternal anatomical and mechanical respiratory adaptations reduce airway resistance, preserve breathing mechanics, minimise the effort of ventilation and thus increase minute ventilation.69
Effect of posture on maternal and fetal cardiovascular dynamics
Supine posture leads to compression of the inferior vena cava by the pregnant uterus, which, in turn, decreases stroke volume, end-diastolic volume index and left ventricular ejection time, with decelerations in maternal HR.70 Being motionless (including standing and certain yoga positions) or exercising in the supine position, may decrease venous return and cause hypotension in 10–20% of pregnant women.70–72 One small study has reported that although uterine blood flow also decreased during supine exercise, this decrease was half that seen during supine rest. If symptomatic, women should avoid the supine position.72 There are no studies on the effect of exercise in the supine position during pregnancy in elite athletes.
Metabolic adaptations in a normal pregnancy
The major energy substrate for growth of the fetoplacental unit is maternal blood glucose, and thus maternal metabolism adapts to supply adequate glucose.73 A cascade of hormonal events increases maternal blood glucose, decreases liver glycogen storage, elevates liver glucose release74 and increases maternal insulin levels.75 This increases insulin resistance in skeletal muscle76 and thus decreases maternal utilisation of glucose in peripheral tissues, which leaves more maternal glucose for fetal use.77 The fetoplacental unit can use as much as 30–50% of the maternal glucose pool in late gestation.78
Changes in responsiveness of the maternal pancreatic β-cell that lead to insulin resistance occur in concert with growth of the fetoplacental unit, as hormones such as human placental lactogen, progesterone, cortisol and prolactin are released.79 Early in pregnancy, maternal body fat is stored, due to the lipogenic action of higher maternal insulin concentrations.80 These adipose stores may provide an alternative energy source for the mother later in pregnancy, and preserve maternal blood glucose for fetal use.81
Thermoregulatory adaptations to pregnancy
The fetal neural tube is formed 35–42 days from the last menstrual period, and therefore exposure to increases in temperature after this time should not affect the risk of neural tube defects.82
During neural tube development, raising body core temperature above 103°F (39°C) can increase the risk of fetal (neural tube defect) abnormalities. Exercising in pregnancy at 60–70% of VO2max in a controlled environment for up to 60 min does not raise core temperature above 38°C.83 Higher body core temperature could be reached during strenuous exercise, such as marathon running, or exercising outdoors in hot and humid weather. However, this has not be evaluated in elite pregnant athletes.
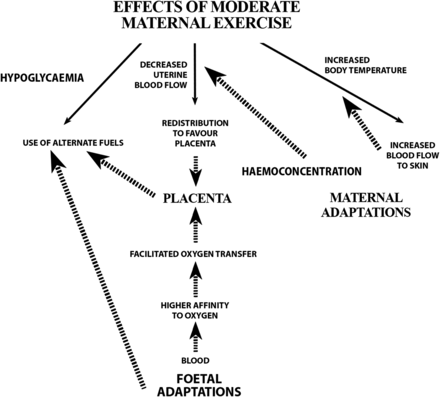
Thermoregulation steadily improves during pregnancy, as reflected by a gradual decline in rectal temperature.84 ,85 As pregnancy advances, a downward shift in body temperature threshold initiates sweating, resulting in evaporative heat loss starting at a lower body temperature.84 The improved heat dissipation at rest may be due to decreased vascular tone, with increased circulation to the skin,86 augmentation of minute ventilation and plasma volume expansion.84 Maternal heat dissipation is vital because fetal metabolism generates heat, and fetal temperature depends on maternal temperature, fetal metabolism and uterine blood flow85 (figure 1).
Flow chart of maternal, placental and fetal adaptations that occur in a low-risk pregnancy to protect the fetus from potential risks of maternal exercise. The solid arrows represent potential effects of maternal exercise. The dashed arrows represent fetal, placental and maternal adaptations that occur in a low-risk pregnancy to counterbalance these potential maternal exercise effects (adapted with permission from Mottola35).
Nutritional requirements for normal pregnancy
Pregnancy increases a woman's requirements for energy, many nutrients and fluid. This permits growth and development of the fetus and deposition of maternal tissue stores.87 Additional energy is needed specifically for development of the fetus, placenta, amniotic fluid, uterus, breasts, adipose tissue, and the increased volumes of blood and extracellular fluid.87 The additional energy need for pregnant women with a mean GWG of 12 kg is estimated to be:
325 MJ (77 700 kcal) in total and 375 kJ/day (90 kcal/day) for the first trimester,
1200 kJ/day (287 kcal/day) for the second trimester, and
1950 kJ/day (466 kcal/day) for the third trimester of pregnancy.88
Energy expenditure is likely to remain high among pregnant elite athletes who continue to train during pregnancy and the total energy intake required will depend on the type, frequency, intensity and duration of the activities performed. An exercising woman can monitor whether she has appropriate energy intake by comparing her weight gain and body mass index (BMI) with the Institute of Medicine (IOM) recommendations (table 2).89
Endurance (including altitude), resistance and flexibility training during pregnancy
Exercise training in pregnant women is influenced by the physiological changes described in the preceding sections. Broadly speaking, women with low-risk pregnancies can undertake the major types of training while pregnant.
Endurance
Among recreational athletes, there were no differences in aerobic fitness (absolute VO2max) tested during the past 2 months of a singleton pregnancy and again 6–8 weeks postpartum.90 In more highly conditioned athletes, a moderate-to-high level of exercise during and after pregnancy may lead to an increase in VO2max in the region of 5–10% after pregnancy.90 ,91 Improved anaerobic working capacity is also better preserved in fitter subjects.92 Taken together, these studies indicate that a woman's aerobic fitness will stay the same or improve slightly during pregnancy if she continues to exercise as her maternal symptoms permit.
Measuring performance during pregnancy: testing VO2max, submaximal tests and HR monitoring
Three standard tests of near maximum exercise capacity (ie, (1) peak VO2 test, (2) true VO2max test83 ,93–95 and (3) tests ending at volitional fatigue)96–100 exist. Harmful events have been reported for neither mother nor fetus after these tests. However, it is not ethical to test pregnant women to ‘failure’. Studies show transient fetal bradycardia when the pregnant elite athlete exercises at above 90% of maximal maternal HR,101–103 but whether these transient fetal HR changes influence neonatal outcomes is unknown. To be appropriately cautious, maximal VO2 testing and exercise above 90% of VO2 max is not recommended, except in highly supervised (research) settings.
An alternative to VO2max testing during pregnancy is to predict target HRs for training from VO2peak tests100 or to predict VO2max from submaximal testing.104–106 Target HR ranges do exist—based on age—that were calculated from fit women at 16–20 weeks of pregnancy who had a VO2 peak of >27.2 mL/kg/min (women aged 20–29 years) and >26.1 mL/kg/min (women aged 30–39 years). The target HR ranges were based on 60–80% of measured VO2 peak values and are listed as 145–160 bpm (women aged 20–29 years) and 140–156 bpm (women aged 30–39 years).100 Given the range of sports in which pregnant elite athletes participate, if desired, these target HRs should be calculated on an individual basis.
Testing with ventilatory analyses (eg, expiratory volume/VO2) is often used to determine target zones for distance and interval training in elite athletes,107 and could provide pregnant women with an alternative form of submaximal exercise testing. If an elite athlete wished to continue training based on ventilatory threshold test results, she can be assured that during mild or moderate exercise the RER (respiratory exchange) is not significantly different in pregnant women than in non-pregnant women.108
During strenuous exercise, the RER rate is lower for the pregnant woman.81 Pregnancy is associated with a mild alkalosis, by increasing respiration at a given metabolic rate.66 The increased respiration causes the woman to expire more CO2 than normal. This makes the RER increase towards 1.0, which, in the non-pregnant athlete, suggests use of carbohydrate stores. However, in the pregnant athlete, this response is not related specifically to metabolism, and this may limit the use of RER to determine substrate and training zones for more intense exercise in pregnant women.109 ,110
Borg scale
During pregnancy, Borg's ratings of perceived exertion (RPE) scale does not correlate strongly with HR.111 HR predicted from RPE is significantly underestimated in the second trimester during walking (ie, actual mean HR is greater than predicted HR by 16 bpm), aerobics classes (15 bpm) and circuit training (18 bpm). In the third trimester, HR while cycling and during aerobics classes are underestimated by a mean of 16 and 11 bpm, respectively, but maximal individual HR underestimations may be up to 54 bpm.111 A woman who uses RPE as a guide may be exercising at a much higher HR than her RPE would suggest. If an elite athlete is trying to keep her HR within a ‘safe’ range, RPE should not be used as the only measure of exercise intensity, particularly from the second trimester. The athlete should measure HR directly.
Intensity of endurance training during pregnancy
Recent studies have shown that most recreational and competitive runners voluntarily reduce their training volume during pregnancy and fewer than one-third continue to run during the third trimester.112 If a woman runs in the third trimester, the intensity of her run is generally reduced.112
Endurance training produces measurable fitness improvement in pregnant women. VO2peak is higher in fit pregnant women (>27.2 and >26.1 mL/kg/min for ages 20–29 and 30–39 years, respectively), than in unfit non-pregnant women (<21.0 and <19.6 mL/kg/min, respectively).100 Athletes of national and international calibre, but representing a range of different sports including rhythmic gymnastics (which is not an endurance sport), had a VO2max range of 38.5–52.6 mL/kg/min at 15–19 weeks of gestation, while maintaining up to 8.4 h/week of vigorous high-volume exercise.91 These findings cannot, however, be directly compared with those of today's non-pregnant female endurance athletes, many of whom have VO2max in the range of 68–76 mL/kg/min.113
Exercise at altitude during pregnancy
Elite endurance athletes regularly train at altitude. Whether the pregnant athlete will (1) benefit from this and (2) not compromise her health or that of the fetus is based on isolated observations and a handful of studies. No study has explored the limits of combined exercise and altitude exposure in pregnancy.114
To test the level of fetal tolerance and responses to an oxygen reduced environment, symptom-limited VO2max exercise tests were conducted in seven healthy pregnant women at 33–34 weeks gestation at sea level, and within a week, also at an altitude of 6000 feet, after a rapid ascent. Although the lowlander participants had some limitations to maximal aerobic capacity, there were no ominous fetal responses.115 There are no studies on pregnant endurance elite athletes exercising at high altitudes (cross-country skiers and runners). The theoretical concern about training at altitude while pregnant is that hypoxia and exercise both decrease blood flow to the uterus, and thus contribute to a decrease in fetal arterial oxygen saturation. Thus, while acknowledging the lack of data, it seems advisable to refrain from high-intensity training regimes at altitudes greater than 1500–2000 m.116
Strength training
Light-to-moderate weight training with free weights or weight machines generally has no adverse health effects during pregnancy.71 ,117–119 Large strength gains have been reported in apparently healthy pregnant women who adopted strength training twice per week for 12 weeks during pregnancy (36% for leg press, 39% for leg curl, 39% for lat pull down, 41% for lumbar extension and 56% for leg extension). Training was associated with a 14% increase in lumbar endurance.120
However, the training doses used in those studies are not comparable to the strenuous weight training performed by some elite athletes. Women who are considering heavy strength training in pregnancy should understand that the Valsalva manoeuvre used during weight training precipitates a rapid increase in blood pressure and intra-abdominal pressure, and therefore may temporarily decrease blood flow to the fetus.37 ,121 ,122 The repercussions to the fetus of these temporary changes remain unknown. In addition, those athletes participating in heavy strength training should acknowledge that large increases in intra-abdominal pressure may harm the pelvic floor support, which may increase the risk of urinary (UI) or anal incontinence (AI) or pelvic organ prolapse (POP) during or after pregnancy.123 Overall, there is sparse knowledge on strenuous strength training in the general pregnant population and no studies on pregnant elite athletes have been conducted.
Flexibility training
Owing to increased levels of relaxin during pregnancy it has been stated that pregnant women are more lax and have more joint instability.124 Neither on the general pregnant population nor on high level exercisers/elite athletes could we find studies measuring range of motion during pregnancy. In addition, no studies were found on the effect of flexibility training during pregnancy.
Sports to avoid during pregnancy
High-risk sports can be divided into those with risk of trauma (eg, from a collision or being hit by something (eg, hockey stick) or falling), and those with physiological risk factors (eg, scuba diving). In relation to maternal trauma, placental abruption leading to acute or chronic fetal hypoxia or death, may occur. Although lots of the available data on this topic have been extrapolated from pregnant women in motor vehicle accidents,125–127 risks also exist in sports where there may be collision or sudden deceleration. An exhaustive list cannot be created, but examples include bobsledding, luge, equestrian (eg, eventing) activities, pole vaulting, ice hockey and downhill ski racing.5
In relation to physiological risk, pregnant women should refrain from diving, because the fetus is not protected from decompression problems and is at risk of malformation and gas embolism after decompression disease.128 ,129
Clinical issues in pregnancy with a focus on exercising women: common complaints and diagnoses—prevention and treatment options
In this section, we highlight the major symptoms of pregnancy and explore medical issues, with a special focus on high-level regular exercisers and elite athletes.
Nausea
Definition
Nausea is a feeling of sickness without actual vomiting. Retching (or dry heaving, without expulsion of stomach contents) has been described as a distinct symptom that has been increasingly measured separately to vomiting and nausea.130 ,131
Morning sickness is a misnomer as it can occur at any time of the day. Pregnant women experience nausea, vomiting and retching mostly in the first 6–12 weeks, but in 20% it can continue up until 20 weeks, or beyond.132 ,133
Hyperemesis gravidarum is characterised by severe and persistent vomiting, intractable vomiting associated with weight loss of more than 5% of pre-pregnancy weight, dehydration and electrolyte imbalance, which may lead to hospitalisation.134 A detailed expert review on nausea and vomiting was published in the BMJ by Jarvis and Nelson-Piercy.135
Prevalence
Nausea and vomiting are common in early pregnancy, with prevalence rates of between 50% and 80% for nausea, and 50% for vomiting and retching.134 ,136 Hyperemesis gravidarum is less common, affecting about 1% of pregnancies.137 No studies on prevalence among elite athletes were found; however, it is intuitive that pregnant elite athletes with severe nausea or vomiting will not be able to train at usual levels.
The exact reasons for nausea, vomiting and retching in pregnancy remain unknown, but they are thought to be associated with rising levels of human chorionic gonadotrophin (HCG) or oestrogen.138 Conditions with higher levels of HCG (multiple pregnancies and molar pregnancies) have been associated with more nausea and vomiting in pregnancy.
Social, physiological and cultural influencing factors have also been studied.139–141 For example, nausea and vomiting in pregnancy may lead to considerable psychosocial stress, through altered family, social and occupational functioning.142–144 Some studies have shown that women with a history of eating disorders are more likely to develop nausea and vomiting in pregnancy.135
Helicobacter pylori seropositivity is also associated with nausea and vomiting in pregnancy.145 In 105 women exposed to H. pylori, there was a dose-dependent link between IgG and severity of hyperemesis gravidarum.146 The clinical implications of this are yet to be established. In patients who do not respond to medical therapy, one might consider investigating for H. pylori as a potentially treatable risk factor.
Differential diagnosis of nausea and vomiting in pregnancy
Hyperemesis gravidarum is a diagnosis of exclusion that requires systematic history-taking and a thorough clinical assessment.135 The differential diagnosis includes other gastrointestinal pathologies including gastritis, appendicitis, cholecystitis and pancreatitis. Other pathologies that should be excluded include urinary tract infections; hyperthyroidism; neurological disorders (eg, migraine); and ear, nose and throat disease (eg, Meniere's disease and vestibular dysfunction).135 Clinicians should be alert for eating disorders such as bulimia, which is more common in elite athletes than in non-athletes and may mimic hyperemesis gravidarum.25
Management of nausea and vomiting in pregnancy
Weight loss, dehydration and electrolyte imbalances are well-recognised complications of severe nausea and vomiting in pregnancy.135 In the most severe cases, fetal growth restriction and prematurity are also recognised complications.147 ,148 In rare instances, Wernicke's encephalopathy due to vitamin B1 deficiency has been documented.149
In view of the potential complications of severe nausea and vomiting, early diagnosis and management is important. This is of particular importance for the elite athlete in training. If a woman has nausea or vomiting of sufficient severity to affect fluid and food intake, the clinician should monitor her weight, examine for signs of dehydration (eg, tachycardia and postural hypotension), test the urine for ketones and consider assessing for signs of hypokalaemia, hypercalcaemia, hypocalcaemia or thyrotoxicosis. Early ultrasonography to identify predisposing factors should be considered (eg, multiple or molar pregnancy).150
Psychological, non-drug-based and drug-based treatments are available for women with nausea and vomiting in pregnancy and hyperemesis gravidarum.135 Dietary support and adequate fluid intake are central to management. Data suggest that women with a high intake of fatty foods have a higher risk of hyperemesis gravidarum and that low-energy high-protein diets are associated with a reduction of nausea and vomiting in pregnancy, compared with a diet high in carbohydrates.151 Women may also benefit from frequent small meals and separating wet from dry intake.
A list of possible antiemetics is given below, for when required. Availability will depend on jurisdiction. Elite athletes should ensure that any drug management is carried out in association with the Governing Body Medical Officer, to prevent using substances that are on the WADA list of banned substances (box 1).
Drugs used commonly in obstetric practice to treat nausea if indicated. These medications were not on the WADA list through 31 December 2015, but elite athletes should always have prescriptions coordinated by their team clinician.135
▸ Doxylamine 10 mg/pyridoxine 10 mg orally, three times daily with higher dose at night.
▸ Cyclizine 50 mg orally, intramuscularly or intravenously, three times daily.
▸ Metoclopramide 10 mg orally, intramuscularly or intravenously, three times daily.
▸ Prochlorperazine 5 mg orally, 12.5 mg intramuscularly or intravenously, three times daily; 25 mg rectally, followed if necessary 6 h later by an oral dose
▸ Promethazine 25 mg orally, at night
▸ Ondansetron 4–8 mg orally, intramuscularly, or by slow intravenous infusion, two to three times daily
Quality rating: high in the general pregnant population, no studies on elite athletes.
If pregnant women fail to respond or weight loss becomes significant, then early admission to the hospital is required for appropriate management.
Nausea and vomiting during pregnancy are common and distressing problems that can compromise quality of life, psychological well-being and performance in elite athletes who otherwise may be competing at that point in the pregnancy.135 The majority experience significant improvement by the early second trimester and can then gradually return to their usual training schedule.
Fatigue
Definition
Fatigue is ‘an overwhelming sustained sense of exhaustion and decreased capacity for physical and mental work’.152
Prevalence
Fatigue is a common complaint throughout pregnancy and during the postpartum period, affecting approximately 90% of pregnancies.153 Factors that contribute to fatigue include living environment, support systems, employment status, socioeconomic status, age, number of children, hours of sleep, exercise and lifestyle.152 ,154 The human body can adapt to fatigue, but sustained fatigue can lead to alterations in quality of life and health status.153 No studies were found to evaluate the rate of fatigue in elite athletes.
Although the majority of fatigue during pregnancy is not related to a pathological process, it is prudent to exclude severe anaemia or hypothyroidism in those with persistent symptoms. Women are considered anaemic in pregnancy if their haemoglobin in the first trimester is <11.0 or <10.5 g/dL after 28 weeks of gestation.150
Neither in the general pregnant population nor in elite athletes were clinical trials found on the effect of exercise to reduce fatigue.
Mental health and well-being
Mental health describes both positive psychosocial well-being and indicators of poor health such as mood disorders, depression and anxiety. Conditions such as eating disorders (anorexia nervosa, bulimia, female triad) are not included in this section, but athletes with these conditions need close follow-up during pregnancy. Poor maternal mental health is associated with poorer pregnancy and birth outcomes, and also poses risks for the offspring later in life.
Depression
Depression is a state of low mood and aversion to activity that can affect a person's thoughts, behaviour, feelings and sense of well-being.155 A clinical diagnosis of depression is based on a diagnostic interview; otherwise the term ‘depressive symptoms’ is used.155
People with depressed mood can feel sad, anxious, empty, hopeless, helpless, worthless, guilty, irritable, ashamed or restless. They may lose interest in activities that were once pleasurable; experience overeating or loss of appetite; have problems concentrating, remembering details or making decisions; and may contemplate, attempt or commit suicide. Insomnia, excessive sleeping, fatigue, aches, pains, digestive problems or reduced energy may also be present.
In the general population, about 6.7% people suffer from depression in a 12-month period,156 and the prevalence is higher in young adults. In pregnancy, the average prevalence of depressive symptoms over seven studies was 22.6%.157 ,158
Because higher levels of physical activity and exercise are generally associated with lower risk of depressive symptoms,159 ,160 it is often presumed that elite athletes will suffer from depressive symptoms less frequently. However, prevalence in elite athletes would suggest no difference in rates of depression from the general population.161 As in the general population, the prevalence of depression is higher in female athletes than in male athletes. Athlete-specific triggers for depression are injury,162 failure in sport performance163 and involuntary termination of the athletic career.164 Among athletes, sports governing bodies and officials, there seems to be a tendency to downplay or ignore psychiatric problems,165 and athletes tend to under-report depressive symptoms and rarely seek help.161 ,166 ,167 Whether this tendency persists in pregnancy is unknown. There are no data on the prevalence of depression among pregnant athletes.
Several studies have shown that depression during pregnancy is associated with an increased risk for poor birth outcomes, including PTB, LBW, IUGR and pre-eclampsia.168 In a meta-analysis of results from studies that used a categorical depression measure, pooled effect sizes were significantly larger (PTB: pooled risk ratio (RR (95% CI)=1.39 (1.19 to 1.61), LBW: 1.49 (1.25 to 1.77) and IUGR: 1.45 (1.05 to 2.02)) than in studies that used a continuous depression measure (1.03 (1.00 to 1.06), 1.04 (0.99 to 1.09) and 1.02 (1.00 to 1.04), respectively).169 ,170 Note that even with these significant relative risks, the majority of women with mental health issues have completely normal pregnancy outcomes. Increased levels of internalising and externalising psychopathology in the children171 as well as difficulties in family relationships172 have been reported.
Anxiety
Anxiety is an emotion characterised by an unpleasant state of inner turmoil, often accompanied by nervous behaviour, such as pacing back and forth, somatic complaints and rumination.173 Anxiety is not the same as fear, which is a response to a real or perceived immediate threat, but is the expectation of future threat.155 Anxiety is often accompanied by muscular tension, restlessness, fatigue and problems in concentration.
In the general population in the Netherlands, the 12-month prevalence of anxiety disorders among women was 12.5% compared with 7.7% in men.174 High levels of anxiety are reported by around one-quarter of women in the first and second trimesters of pregnancy.175 However, no consensus exists on diagnostic instruments or cut points for identifying individuals with high anxiety levels, so prevalence rates vary between studies. No data are available for pregnant athletes, and there are limited data on anxiety in non-pregnant athletes. In Australia, one study has reported that 12% of female athletes had high anxiety scores.176
Women may also suffer from specific pregnancy-related anxiety, such as fear of having a handicapped child, fear of giving birth, and anxiety about their own changing body and appearance.177 These pregnancy-related anxieties are not associated with general anxiety or depressive feelings,177 and are reported by 14.4% of pregnant women.178 Fear of giving birth has been reported by 8% of women in a general pregnant population.179 Whether athletes suffer more or less from pregnancy-related anxieties is unknown, but they might have anxieties about being able to return to their sporting career, in the same way as some women have anxiety about returning to work.180
Prevention and treatment of depression and anxiety during pregnancy
Pharmaceutical treatment of depressive symptoms with antidepressants, such as selective serotonin reuptake inhibitors, is common in the general population. The decision to use antidepressants in pregnancy is based on the mental health needs of the mother during pregnancy and the postpartum period balanced against any theoretic concern for the fetus. That being said, most antidepressants are not contraindicated in pregnancy.181 Any medication used by an elite athlete should fit within the WADA guidelines.182
Cognitive behavioural therapy, an effective preventive and treatment option for both depression and anxiety outside pregnancy,183 ,184 is also effective in pregnancy and the postpartum period.185 A 2014 systematic review and meta-analysis found some evidence to support the effectiveness of exercise in the prevention and treatment of depression in pregnancy, based on the findings of six intervention studies of ‘low-moderate’ quality.186 However, these studies were not undertaken in elite athletes.
Gestational weight gain
Pre-term birth—defined as the amount of weight gained from conception to delivery—when extreme, may affect the health and well-being of the mother and infant, 89 187 particularly in the second and third trimester, GWG is an important determinant of fetal growth.89 Pregnant women who gain little weight and who are below IOM recommendations, are more likely to give birth to a SGAS infant, especially normal weight and underweight women.187 ,188 In addition, low GWG is associated with PTB and failure to initiate breast feeding.188 ,189 On the other hand, women who gain above the recommended amount have increased risk of GDM and hypertensive disorders, prolonged labour, caesarean section, macrosomia and large for gestational age infants, as well as postpartum weight retention and later overweight/obesity.190–192 Both GWG above and below recommendations could occur in elite athletes, and may be of concern for pregnancy outcomes as well as for return to sport.
Current recommendations for GWG were issued in 200989 and are based on pre-pregnancy BMI categories (table 2). Women pregnant with twins are given separate recommendations, ranging from 16.8 to 24.5 kg (37–54 lbs) for normal weight women, 14.1 to 22.7 kg (31–50 lbs) for overweight women and 11.3 to 19.1 kg (25–42 lbs) for obese women. There are no recommendations specifically for elite athletes.
Recommendations for total weight gain range in singleton pregnancies by pre-pregnancy body mass index (BMI) (Institute of Medicine)89
Description of GWG
GWG is derived from both maternal and fetoplacental factors, including increased blood volume (3–4 lbs or 1.4–1.8 kg), uterus (2 lbs or 0.9 kg), increased extracellular fluid (3–4 lbs or 1.4–1.8 kg), larger breasts (1–2 lbs or 0.4–0.9 kg), fat stores for breast feeding (5–8 lbs or 2.3–3.6 kg), the baby (7–8 lbs or 3–3.6 kg), placenta (1–1.5 lbs or 0.5–0.7 kg) and amniotic fluid (2 lbs or 0.9–1 kg).88 ,89
The rate of weight gain is usually lowest (0.5–2.0 kg in total) in the first trimester, highest in the second trimester (on average below 0.50 kg/week) and relatively constant or somewhat decreasing towards the end of the third trimester.88 ,89
Adherence to weight gain recommendations
Recent data from the USA show that approximately 15–20% of women gained below, 26–36% gained within and 42–57% gained above the IOM recommendations relating to GWG.193–197 Hence, in a high percentage of pregnant women, weight gain is outside the current guidelines.
The role of exercise and GWG
In the general obstetric population, observational studies have shown inconsistent results with respect to relationships between exercise/physical activity and GWG. Some studies have shown that a decline in exercise from pre-pregnancy levels was significantly related to higher GWG.119 ,198 Another longitudinal cohort study showed that women meeting the physical activity guidelines (≥30 min per day) had 29% (95% CI 0.57% to 0.88%) lower odds of gaining above the IOM recommendations than did inactive women.199 Stuebe et al200 ,201 also reported that mid-pregnancy walking and moderate-intensity exercise, in accordance with present guidelines, was inversely associated with the risk of excessive GWG.
Of potential relevance for athletes, studies have shown that exercise performance influences neither the rate of early pregnancy weight gain nor subcutaneous fat deposition (skinfold thickness), with both decreasing in late pregnancy. Still, overall pregnancy weight gain was within the normal range.202–204 Also, higher pre-pregnancy physical activity levels have been associated with less GWG,205 which is relevant for the athlete. Only one observational study reporting GWG in elite athletes was found.206 In this retrospective study of 40 Norwegian elite athletes and age-matched controls with normal pre-pregnancy BMI, self-reported mean GWG was lower in the athletes than in the controls (13.9 SD 6.9 kg vs17.5 SD 9.1 kg, p=0.06).
Prevention and treatment
Several randomised controlled trials (RCTs) have investigated the effect of exercise on GWG and results differ.8 ,207–230 However, none of these RCTs included competitive athletes. Kardel91 investigated the effect of a high-volume and medium-volume training regime in pregnant elite athletes (n=41), and in neither fat percentage nor GWG was a significant difference between the two training groups reported. Studies investigating the impact of training volume on GWG throughout pregnancy (early vs late) and the effect on the growing fetus are warranted, but difficult to conduct. Quality rating: low, no RCTs in elite athletes. As long as symphysis fundal heights are consistent with gestational age, more frequent ultrasound assessments are not required for elite athletes.
Pre-eclampsia
Definition
Pre-eclampsia represents a spectrum of hypertensive disorders complicated by proteinuria presenting exclusively during pregnancy. The spectrum includes those who develop minor versions of the disorder near term as well as those who develop severe versions that require significantly premature delivery, the only known cure for the disorder. In addition to hypertension and proteinuria, the most severely affected patients may also develop evidence of end-organ effects manifested by haemolysis, abnormal liver or renal function, clotting disorders, and thrombocytopaenia. Severe cases may also present with fetal growth restriction, increasing the risk of stillbirth.231 A detailed expert review on pre-eclampsia was published by Stocks.232 The level of the quality of the body of evidence for this topic is moderate.
Prevalence
Pre-eclampsia affects between 2% and 7% of women having their first child and, of these, 75% will present with signs and symptoms near term.231 Multiparous women have a lower risk of pre-eclampsia at 1.7%.233 Compared with Caucasian women, African, African-American and Latina women have an increased risk of pre-eclampsia.234 ,235 The overall rate of pre-eclampsia has been rising over the past 20 years, due to an increase in two common risk factors, obesity and diabetes.236 There are no studies on prevalence of this condition in elite athletes.
Risk factors
The cause and exact mechanisms for pre-eclampsia remain unclear, but several risk factors have been identified (table 1). Lack of physical activity should be added to this list. Some of these risk factors would generally be considered to be less prevalent in the elite athlete population than in the general population due to their tendency to younger age and low rates of obesity (box 2).
Risk factors for pre-eclampsia
Nulliparity
▸ Pre-eclampsia in a previous pregnancy
▸ Maternal age >40 years
▸ Assisted reproductive technologies
▸ Obesity or excessive weight gain in pregnancy
▸ Mother or sister with history of pre-eclampsia
▸ Maternal low birth weight or premature delivery
▸ Heritable thrombophilias
▸ Chronic hypertension
▸ Type 1 diabetes mellitus
▸ Renal disease
▸ Multifetal gestation
▸ Cocaine or amphetamine use
Once pre-eclampsia is diagnosed (box 3), most patients will undergo investigations to determine whether there have been associated end-organ effects. The typical investigations include a blood smear, haemoglobin and haematocrit count, platelet count, liver transaminases, creatinine, uric acid, and clotting studies. By performing these investigations, care givers can exclude the HELLP (haemolysis, elevated liver enzymes, low platelets) syndrome, one of the most severe versions of pre-eclampsia. In addition, evaluation of the fetus is required to assess growth and well-being.
Diagnosis
Pre-eclampsia is diagnosed by the combination of new hypertension >140/90 mm Hg and proteinuria after 20 weeks of gestation. Proteinuria should be suspected when there is a urinary dipstick of >1+. This should be quantified using either a 24 h urine protein collection of >0.3 g/day, or spot urinary albumin of >30 mg/mmol of creatinine. Pre-eclampsia is considered severe if any of the following are present: systolic blood pressure >160 mm Hg, diastolic blood pressure >110 mm Hg, severe headache or visual disturbances, right upper quadrant/epigastric pain, nausea and vomiting, proteinuria >5 g/day, oliguria <500 mL/24 h, pulmonary oedema or fetal growth restriction.231 ,237
Treatment
There is no specific treatment for pre-eclampsia. Management will depend on the severity of hypertension, evidence of end-organ effects, assessments of fetal well-being and, most importantly, gestational age. Most commonly, induction of labour will be offered to term patients with mild pre-eclampsia, whereas, the same patient, when at less than 34 weeks of gestation, may be placed on antihypertensives and given corticosteroids to advance fetal lung maturity, with an attempt to achieve a greater gestational age at birth. In contrast, despite even a gestational age <28 weeks, patients with the most severe forms of pre-eclampsia will require early delivery as there is no other cure for the rapidly worsening hypertension and end-organ effects. The most severely affected patients will also be treated with magnesium sulfate before and after delivery, to prevent the development of seizures (eclampsia).231 ,237
Mechanisms through which exercise might reduce the risk of pre-eclampsia
Weissgerber et al238 proposed four mechanisms by which exercise might reduce the rate of pre-eclampsia. These include enhanced placental growth and vascularity; prevention and/or reduction of oxidative stress; reduction of inflammation; and correction of endothelial dysfunction. Each of these potentially interactive factors promotes endothelial dysfunction, which leads to late stage symptoms of pre-eclampsia.236 ,239 They also suggest that, for maximum effect on these mechanisms, exercise should be started in the first trimester.236 ,238
The role of exercise in preventing pre-eclampsia
Most studies evaluating the effect of exercise on the rate of pre-eclampsia demonstrate benefit. In their 2014 meta-analysis,233 Wolf et al233 included 11 observational studies evaluating the impact of leisure time physical activity on the rate of pre-eclampsia. No RCTs were included. The amount of physical activity was self-reported in all studies and included leisure activities such as gardening, walking, running and other aerobic exercises.
When evaluating the impact of strenuous exercise during pregnancy on the rate of pre-eclampsia, the studies were inconsistent, with four showing a reduction in pre-eclampsia and the others showing no benefit. One study identified an increased risk of severe pre-eclampsia in those who exercised more than 270 min/week. None of the studies showed any benefit of light or moderate leisure time physical activity on the rate of pre-eclampsia.233
Also, in 2014, Aune et al234 published their systematic review and meta-analysis of data from 15 cohort and case–control studies. They reported a 40% reduction in pre-eclampsia for those women who performed strenuous exercise prior to pregnancy, but with no further improvement with durations >5–6 h of exercise per week. For studies that reported exercise before 24 weeks gestation, they identified a 21% reduction in pre-eclampsia, with greater benefit in those who exercised at high intensity (45% reduction) than in those who exercised at low intensity. Compared with non-exercisers, women who exercised before and during pregnancy had a 36% reduction in pre-eclampsia.234
Only one small RCT has evaluated exercise and risk of pre-eclampsia.240 There was a significantly lower rate of pre-eclampsia in the stretching exercise group (2.6%, N=38) than in the walking group (14.6%, N=41). The authors also identified a significantly higher rate of transferrin, an antioxidant marker, in the stretching group, and suggested that regular stretching produces more endogenous antioxidants. Quality rating: low. No studies have evaluated the effects of exercise on the rate of pre-eclampsia in elite athletes.
Gestational hypertension
Gestational hypertension is newfound hypertension >140/90 after 20 weeks gestation without proteinuria.231
Prevalence
Approximately 6% of women will develop gestational hypertension and 25% of these will go on to develop pre-eclampsia, which is discussed in another section of this document.231 ,241 There are no studies in elite athletes.
Risk factors
Risk factors for gestational hypertension include nulliparity, advanced maternal age, obesity, diabetes and renal disease.242 ,243
Diagnosis
Gestational hypertension is diagnosed after 20 weeks gestation in women with no history of hypertension and no proteinuria when the blood pressure is >140/90 on at least two occasions, at least 4 h apart.231
Treatment
Treatment of gestational hypertension is dependent on gestational age. Given the frequency that gestational hypertension transitions to pre-eclampsia, most women at term will be offered induction of labour to avoid the possibility of this more serious hypertensive complication of pregnancy. Women who are preterm are commonly started on antihypertensive medications and the frequency of assessments is increased to look for evidence of pre-eclampsia, or fetal growth restriction.231
The role of exercise in the prevention of gestational hypertension
The large majority of studies evaluating the impact of exercise on hypertensive complications of pregnancy have used the rate of pre-eclampsia, rather than the milder gestational hypertension, as the outcome.
Two epidemiological studies have evaluated the impact of exercise exposure before and during pregnancy on the rate of gestational hypertension. One reported a 70% reduction in gestational hypertension among Hispanic women who exercised before pregnancy; however, the upper confidence limit was 1.0.244 The other found no effect of exercise before or during pregnancy on the rate of gestational hypertension.245 However, a recent large RCT showed that women who initiate structured exercise early in pregnancy are three times more likely to prevent gestational hypertension, are 1.5 times more likely to prevent excessive GWG and are 2.5 times more likely to prevent macrosomic or large babies.246 Quality rating for prevention: moderate, no studies in elite athletes.
To date, no study has evaluated exercise as a therapy for gestational hypertension (quality rating: very low) and no studies of gestational hypertension have been conducted with elite athletes.
Oedema
Oedema refers to the accumulation of excessive watery fluid in cells, tissues, or serous cavitites. It is very common for pregnant women to experience dependent oedema, especially during the third trimester. It is also linked to pre-eclampsia and hypertension. The physiological explanation for dependent oedema is related to the substantial increase in blood volume, vessel permeability and extracellular fluids, as well as the obstructive pressure of the uterus on the vena cava. There are no published studies suggesting prenatal exercise as a cause of dependent oedema.
Hydrotherapy has been suggested as a treatment for dependent oedema by increasing hydrostatic pressure and causing diuresis.247 Kent et al248 investigated the impact of hydrotherapy on dependent oedema. Eighteen healthy women between 20 and 33 weeks were evaluated for 30 min segments standing on land, standing in water to the axilla and during a low-intensity water aerobics class with water to the axilla on separate days and in a random order. Diuresis posttherapy was higher for water aerobics (187 mL) and static immersion (180 mL) than for standing on land (65 mL, p=0.01). Hartmann and Huch249 also showed that a single session of immersion exercise significantly decreased the volume and circumference of both legs. Level of evidence: low. No studies in elite athletes.
Gestational diabetes
Definition
GDM is glucose intolerance with onset or first recognition during pregnancy.
Prevalence
The prevalence of GDM is increasing, affecting almost 10% of pregnancies, a reflection of the global obesity epidemic.250 ,251 Women diagnosed with GDM are at high risk for future diabetes, with approximately 50% of women developing type 2 diabetes within 5 years of delivery. It has been estimated that, in some populations, women with a history of GDM may account for up to one-third of diabetes cases among parous women.252 Women with GDM represent a heterogeneous group. Some women have unrecognised pre-existing type 2 diabetes and a small number have type 1 diabetes, with onset during pregnancy; however, the majority are first diagnosed during pregnancy.250
There are no prevalence studies of GDM among elite athletes. However, women who are most active before253 ,254 and during pregnancy255 are at lower risk of developing GDM.256 ,257
Pathophysiology
During pregnancy, insulin resistance increases and euglycaemia is maintained through a compensatory increase in insulin secretion. GDM appears to be a failure to compensate with increased insulin secretion. As the increase in insulin resistance is greatest in the third trimester, GDM usually develops during this time. Therefore, screening for GDM typically occurs around 24–28 weeks gestation.258 ,259
Diagnosis of GDM
There has been much debate about which—universal or selective—screening of pregnant women for GDM is more appropriate. Selective screening for those with the highest risk has been recommended, especially in low-risk countries. As the prevalence of GDM increases, there is a tendency towards universal screening, as 40% of GDM cases remain undiagnosed using selective screening.260 ,261 The fasted oral glucose tolerance test is the diagnostic test, although criteria vary around the world.
Risk factors for the development of GDM include obesity, older age, family history, previous history of GDM, or poor obstetric outcomes, ethnicity, polycystic ovarian syndrome and, as recently noted, hypertension.250 ,258 Lack of physical activity before and during pregnancy may lead to obesity and is an important risk factor.262–267
GDM is strongly associated with adverse prenatal and postnatal outcomes, and is related to long-term and short-term morbidity in both the offspring and mother. Adverse infant outcomes include macrosomia (babies born >4.0 kg), hypoglycaemia, erythaema, hypocalcaemia, jaundice and birth trauma.250 ,268 Later in life, these children are more likely to become obese, have abnormal glucose tolerance and to develop diabetes in adolescence or early adulthood. Women with GDM experience an increased risk of developing pre-eclampsia, infection and postpartum haemorrhage, and are more likely to develop overt diabetes, usually type 2, after pregnancy.250 ,251
Given the risk factors, high-level exercisers and elite athletes would be expected to have reduced risk for developing GDM.269 Therefore, we do not review intervention studies in this report.
Musculoskeletal complaints
Pelvic girdle pain and low back pain
Definition
Pelvic girdle pain (PGP) is pain experienced between the posterior iliac crest and the gluteal fold, particularly in the vicinity of the sacroiliac joints.270
PGP often arises in relation to pregnancy, with pain related to the pelvic musculoskeletal system and not from gynaecological or urological disorders. After the exclusion of lumbar causes, diagnosis of PGP can be made by reproducing the pain/functional impairments related to PGP, using specific clinical tests.270
Although similar and overlapping features may be ascribed to low back pain (LBP) and to PGP, during pregnancy, PGP appears to have more impact on disability than does LBP.38 ,270–273 PGP disorders have a characteristic clinical presentation, and with this comes a need for specific management.273–275 Pregnancy-related LBP and PGP are common in many countries, regardless of socioeconomic factors,276–278 and have a negative effect on daily activities, exercise and training.279
Prevalence
The prevalence rate of pregnancy-related LBP and PGP is estimated to be about 50% during pregnancy.280 An estimated 20–25% of all pregnant women suffer from PGP sufficiently seriously to require medical help.270 ,280 Only one study has investigated PGP and LBP in elite athletes, reporting a prevalence of 29.6% for PGP and 18.5% for LBP during pregnancy, similar to non-athletic controls.206 Previous LBP, previous LBP and/or PGP during or after pregnancy, strenuous work and previous trauma to the pelvis, are commonly identified risk factors.270 ,280
Aetiology and pathogenesis
The aetiology and pathogenesis of PGP is uncertain and probably multifactorial. The pelvis transfers load from the trunk to the legs and this requires a stable pelvis. Overload of the ligaments of the pelvis may be a result of impaired load transfer during activities and hence have an influence on PGP.281–283 The sacrospinous and the long dorsal sacroiliac ligament are both thought to be a possible source of pain in PGP.284–288 The changes in spinal curvature during pregnancy as well as frequent or sustained pain-provoking postures might influence the pelvic ligaments, causing pain. It is probably important that elite athletes are aware of their body positions while pregnant to avoid unnecessary load and stress on joint, ligaments and muscles, during exercise and daily activities.
Treatment and prevention
Twenty-six RCTs that examined the effects of a variety of interventions for LBP and PGP during pregnancy were included in an updated Cochrane review.289 Based on evidence of moderate quality, this review suggests that different types of exercise and acupuncture significantly reduce evening PGP or lumbopelvic pain more than normal care alone. According to the European guideline for PGP, individualised exercises to treat PGP in pregnancy are recommended.270
There is limited knowledge on how to prevent LBP and PGP in pregnancy,289 with lack of evidence of a positive effect of group training,290–293 and inconsistent findings on whether regular physical activity before pregnancy can reduce the risk of LBP and/or PGP during pregnancy.294 ,295 Women who reported high-impact exercise 3–5 times per week before pregnancy had a 14% lower risk of developing severe PGP in pregnancy compared with non-exercisers.296 In a longitudinal cohort study, a greater loss of physical condition seems not to be a cause but rather a consequence of LBP and/or PGP in pregnancy,297 hence knowledge about type of exercise and dose is needed. Quality rating: moderate. No studies on prevention or treatment of LBP and PGP in pregnant elite athletes were identified.
Pelvic floor dysfunction
Definitions
Pelvic floor dysfunction (PFD) is defined as the presence of symptoms of UI or AI, POP, sensory or emptying abnormalities of the lower urinary tract, defaecation dysfunction, sexual dysfunction and pelvic floor pain syndromes. The conditions can present separately or coexist.298 UI is defined as a complaint of involuntary passing of urine.299 The most prevalent form of female PFD is stress UI (SUI)—‘the complaint of involuntary leakage on effort or exertion (eg, sporting activities), or on sneezing or coughing’.299 Urgency UI (UUI) is the complaint of involuntary passing of urine associated with urgency.299 AI is ‘involuntary passing of faeces or flatus’, while faecal incontinence is limited to involuntary passing of faeces (solid or liquid).299 The definition of anatomical POP is ‘the descent of one or more of the anterior vaginal wall, posterior vaginal wall, the uterus (cervix), or the apex of the vagina (vaginal vault or cuff scar after hysterectomy)’.299 The presence of any such sign should be correlated with relevant POP symptoms. POP ranges from asymptomatic minor changes in vaginal support, common after childbirth, to severe vaginal bulging for which women choose treatment.
Prevalence
The prevalence of UI in nulliparous women and in female elite athletes is high.300 ,301 Prevalence rates range between 28% and 80% during sporting activities in female elite athletes.301 UI occurs most commonly in sports involving high-impact activities such as trampolining and gymnastics. While athletes report that they feel embarrassed about the condition and that it may affect performance, in one study, 84% had never spoken with their coaches or healthcare providers about UI.302 In a retrospective study on Norwegian elite athletes who had given birth, 12.9% reported SUI the year before the birth and 18.5% during pregnancy. These rates of prevalence did not differ from those in a matched control group.206
AI affects one in four primiparous women during the third trimester.303 ,304 In one study, the prevalence of AI, generally limited to flatus incontinence, was higher in women (aged 18–40 years) who exercised more than 8 h/week (14.8%) than in a comparison group (4.9%, p=0.001).305 In the retrospective study of elite athletes, none recalled AI during pregnancy or after childbirth.206
Among 116 nulliparous female US Military Academy cadets, those who attended paratrooper training were significantly more likely to demonstrate stage II POP on examination after 6 weeks than those who did not (RR=2.72, 1.37<RR<5.40; p=0.003).306
No prospective studies of POP in elite female athletes (pregnant or non-pregnant) were found.
Aetiology and risk factors for PFDs
Risk factors for PFDs include pregnancy and childbirth-related factors (injuries to peripheral nerves, connective tissue and muscles), heritage and ethnicity, obesity, behavioural factors, ageing, and strenuous work. Vaginal birth is the strongest risk factor for POP in women of all ages, while vaginal birth increases the risk for UI by about two-fold in younger and middle-aged women, but no longer plays a role in older women.123 ,300 ,307
DeLancey et al308 presented an integrated life span model of causal factors of PFD. Phase I comprises predisposing factors, which include genetic construction, nutritional factors and socialisation. Phase II comprises inciting factors, which include predisposing maternal–fetal factors such as pelvic floor shape and size, macrosomic infant, fetal head position, effects of obstetric interventions such as use of forceps, prolonged second stage and occipit posterior position, all leading to potential injury (muscle, connective tissue and nerve avulsion/compression). Phase III comprises intervening factors, which include variation in normal ageing of muscles, nerves and connective tissue; increased stress to the pelvic floor (occupational lifting, strenuous activity, obesity or chronic cough); and factors leading to weakening of the support tissues (chronic steroid use or disuse atrophy of muscles).
Aetiology and risk factors for UI in elite athletes
Bø et al301 found that women exercising ≥3 times per week at gestational week 37 had a significantly larger levator hiatus area than did non-exercising women. However, these women were not elite athletes. In a study of college athletes, Nygaard et al309 found no significant association between UI and amenorrhoea, weight, hormonal therapy or duration of athletic activity. In a study of former US Olympians, among factors such as age, BMI, parity, Olympic sport group and incontinence during Olympic sport 20 years ago, only current BMI was significantly associated with regular SUI or urgency incontinence symptoms.310 Bø and Sundgot-Borgen311 reported that significantly more elite athletes with eating disorders had symptoms of both SUI and UUI. Eliasson et al312 showed that incontinent trampolinists were significantly older (16 vs 13 years), had been training longer and more frequently, and were less able to interrupt the urine flow stream by voluntarily contracting the pelvic floor muscle, than the non-leaking group.
Prevention and treatment
Devices that involve external urinary collection, intravaginal support of the bladder neck or blockage of urinary leakage by occlusion are available,313 and some have reportedly been effective in preventing leakage during physical activity.314 For smaller leakage, specially designed protecting pads can be used during training and competition. No RCTs have evaluated the effect of preventive devices in elite athletes.
According to the most recent Cochrane review, in the general population, pregnant women without UI randomly assigned to intensive antenatal pelvic floor muscle training were less likely to report UI up to 6 months after delivery than were controls who received usual care (about 30% less; RR 0.71, 95% CI 0.54 to 0.95, combined result of five trials).315 As several studies have found that >30% of women are not able to perform a correct PFM contraction at their first consultation, thorough instruction of how to perform a contraction and assessment of ability to contract is vital.316 In studies with favourable results, the women have performed supervised PFM training in combination with home exercise.315
Prevention/treatment
No studies were found on prevention or treatment strategies for diastasis recti abdominis during pregnancy, either in the general pregnant population or in elite athletes. Quality rating: high in the general population. There are no studies of interventions to prevent or reduce PFD during pregnancy in female elite athletes.
Diastasis recti abdominis
Definition
Diastasis recti abdominis (DRA) is an impairment with midline separation of the two rectus abdominis muscles along the linea alba.317
Prevalence
Prevalence rates (with and without protrusion/hernia) during pregnancy vary between 27% and 100% in the second and third trimester.318 ,319 There are no prevalence studies among elite athletes during pregnancy.
DRA is diagnosed by measuring the distance between the median borders of rectus abdominis (inter-rectus distance); measurement methods in use are palpation with fingerbreadths, caliper or ultrasound.320 To date, there is no consensus on where to measure the distance along the linea alba, or on the cut-point for diagnosing the condition.319
Aetiology and risk factors
The aetiology of DRA is not clear. While some believe that DRA affects abdominal muscle strength or spinal stability during pregnancy, we identified no data on DRA and LBP or PGP during pregnancy, and found no studies testing the theory that DRA can be prevented or treated with abdominal or other exercises during pregnancy. A recently published systematic review concluded that there is an urgent need for more research on the prevalence, risk factors, prevention and treatment of the condition.321 Female athletes usually train the abdominals regularly and anecdotally have stronger abdominal muscles than the general population. Whether this is a protective factor or a risk factor for development of DRA in pregnancy is not known. Quality rating: very low. There is no evidence to guide elite athletes on abdominal training during pregnancy.
Other parts in the 5-part series
In addition to this Part 1, four other parts of IOC expert group meeting will be published in future issues of BJSM. Part 2: Effect of having exercised (physical fitness) on labour and neonatal/fetal outcomes. Part 3: Guidance on returning to exercise in the the postpartum period. Part 4: Research directions. Part 5: Recommendations for health professionals and active women.
References
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
Footnotes
Contributors KB conceived the idea and consulted all the other authors in the development of the consensus meeting. All the authors prepared material and submitted it in advance of the in person meeting in Lausanne. All the authors contributed materially to drafting the various versions of the document and approved the final version.
Funding IOC, 10.13039/501100003965, Internal Funds For Consensus Meetings.
Competing interests None declared.
Provenance and peer review Not commissioned; internally peer reviewed.