Article Text
Abstract
Sudden cardiac death (SCD) is the leading cause of mortality in athletes during sport. A variety of mostly hereditary, structural or electrical cardiac disorders are associated with SCD in young athletes, the majority of which can be identified or suggested by abnormalities on a resting 12-lead electrocardiogram (ECG). Whether used for diagnostic or screening purposes, physicians responsible for the cardiovascular care of athletes should be knowledgeable and competent in ECG interpretation in athletes. However, in most countries a shortage of physician expertise limits wider application of the ECG in the care of the athlete. A critical need exists for physician education in modern ECG interpretation that distinguishes normal physiological adaptations in athletes from distinctly abnormal findings suggestive of underlying pathology. Since the original 2010 European Society of Cardiology recommendations for ECG interpretation in athletes, ECG standards have evolved quickly, advanced by a growing body of scientific data and investigations that both examine proposed criteria sets and establish new evidence to guide refinements. On 26–27 February 2015, an international group of experts in sports cardiology, inherited cardiac disease, and sports medicine convened in Seattle, Washington (USA), to update contemporary standards for ECG interpretation in athletes. The objective of the meeting was to define and revise ECG interpretation standards based on new and emerging research and to develop a clear guide to the proper evaluation of ECG abnormalities in athletes. This statement represents an international consensus for ECG interpretation in athletes and provides expert opinion-based recommendations linking specific ECG abnormalities and the secondary evaluation for conditions associated with SCD.
- Athlete
- Sudden cardiac arrest
- Sudden cardiac death
- Cardiovascular
Statistics from Altmetric.com
Introduction
Cardiovascular-related sudden death is the leading cause of mortality in athletes during sport and exercise.1–3 The majority of disorders associated with an increased risk of sudden cardiac death (SCD), such as cardiomyopathies and primary electrical diseases (channelopathies), are suggested or identified by abnormalities present on a resting 12-lead ECG. Interpretation of an ECG in athletes requires careful analysis to properly distinguish physiological changes related to athletic training from findings suggestive of an underlying pathological condition. Whether used for the evaluation of cardiovascular-related symptoms, a family history of inheritable cardiac disease or premature SCD, or for screening of asymptomatic athletes, ECG interpretation is an essential skill for all physicians involved in the cardiovascular care of athletes.
The 2015 summit on ECG interpretation in athletes
On 26–27 February 2015, an international group of experts in sports cardiology, inherited cardiac diseases and sports medicine convened in Seattle, Washington, to update contemporary standards for ECG interpretation in athletes through development of an international consensus. This summit meeting served as the foundation for subsequent work done by the larger writing group that ultimately generated this document. The goals of the summit meeting were to: (1) update ECG interpretation standards based on new research and up to date evidence and (2) develop a clear guide to the appropriate evaluation of ECG abnormalities for conditions associated with SCD in athletes.
The standards presented were developed with consideration of ECG interpretation in the context of an asymptomatic athlete aged 12–35 years. An athlete is defined as an individual who engages in regular exercise or training for sport or general fitness, typically with a premium on performance, and often engaged in individual or team competition. The prevalence of specific ECG findings in athletes may vary based on age, sex, ethnicity, type of sport and level of conditioning. Training-related physiological changes are more common in athletes exercising intensively at least 4–8 hours per week; thus prudent application of the criteria should occur in individuals at lower levels of regular exercise. Novel to these standards are specific considerations presented for young adolescent athletes age 12–16 years, as well as for older athletes ≥30 years where the prevalence of occult coronary artery disease sharply increases. In the presence of cardiac symptoms or a family history of inherited cardiovascular disease or premature SCD, the interpretation standards may require modification.
The recommendations presented in this statement were developed with thoughtful attention to balance sensitivity and specificity, while maintaining a clear and practical checklist of findings to guide ECG interpretation for physicians and the appropriate evaluation of ECG abnormalities. A summary of consensus recommendations from this panel is presented in figure 1, table 1 and table 2. Physicians may choose to deviate from these consensus standards based on their experience or practice setting and according to the individual characteristics of the athlete. Ideally, the evaluation of ECG abnormalities is performed in consultation with a specialist with knowledge and experience in training-related cardiac adaptations and disorders associated with SCD in young athletes.
International consensus standards for ECG interpretation in athletes. AV, atrioventricular; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; PVC, premature ventricular contraction; RBBB, right bundle branch block; RVH, right ventricular hypertrophy; SCD, sudden cardiac death.
International consensus standards for ECG interpretation in athletes: definitions of ECG criteria
Evaluation of ECG abnormalities in athletes
Distinguishing normal from abnormal
A challenge in the interpretation of an athlete’s ECG is the ability to accurately differentiate findings suggestive of a potentially serious cardiovascular disorder from benign physiological adaptations occurring as the result of regular, intense training (ie, athlete’s heart). Several reports have outlined contemporary ECG criteria intended to distinguish normal ECG findings in athletes from ECG abnormalities requiring additional evaluation.4–10
Evolution of ECG interpretation standards
Over the last decade, ECG interpretation standards have undergone several modifications to improve the accuracy of detecting potentially life threatening cardiac conditions in young athletes while also limiting false-positive results. In 2005, criteria for an abnormal ECG were defined by the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology (ESC).11 In 2010, driven by new research and large-population clinical investigations,12 13 the ESC updated recommendations for interpretation of an athlete’s ECG in a landmark statement which pioneered ‘modern’ ECG interpretation standards.5 The 2010 ESC criteria were the first to divide ECG findings into two groups—common and training-related (group 1) versus uncommon and training-unrelated (group 2)—based on the prevalence of ECG findings, relation to exercise training, and association with pathological conditions associated with SCD requiring further clinical investigation to confirm (or exclude) underlying cardiovascular disease.5 Since then, other investigators and international panels have proposed updated guidelines for ECG interpretation in young athletes.6 9 14 In 2012, leading sports medicine, inherited cardiac disease specialists, and sports cardiology physicians with expertise in the cardiovascular evaluation of young competitive athletes convened in Seattle, Washington, to redefine contemporary standards and develop an online training course (http://learning.bmj.com/ECGathlete) for ECG interpretation in athletes.10 15–17
Each revision of the ECG standards has improved specificity while maintaining the sensitivity for ECG-detectable pathological conditions associated with SCD. For example, in a study of 1078 elite Australian athletes, the false-positive rate decreased from 17% using the 2010 ESC criteria to 4.2% with the Seattle criteria, with no change in sensitivity.18 In another study of 2017 high school athletes from the U.S., the Seattle criteria produced a low false-positive rate (2.8%) with 100% sensitivity for SCD-associated conditions.19 In a study of 1417 high school, college and professional athletes comparing three published ECG interpretation algorithms for athlete preparticipation screening, the proportion of abnormal ECGs declined from 26% to 8.1% to 5.7% using the ESC, Stanford and Seattle criteria, respectively.20
Recent research has directed additional revisions to ECG interpretation standards that further lower the false-positive rate.21 22 A ‘refined’ criteria set was investigated in an instrumental study examining 4297 white and 1208 black elite athletes, and 103 athletes diagnosed with hypertrophic cardiomyopathy (HCM).23 The total ECG abnormal rate declined dependent on the criteria applied—21.5% (ESC), 9.6% (Seattle) and 6.6% (refined)—while all three criteria identified 98.1% of athletes with established HCM.23 Of the 5505 athletes screened, 15 (0.47%) were diagnosed with conditions associated with SCD, including 5 cases of HCM, 5 cases of Wolff-Parkinson-White (WPW), 3 cases of long QT syndrome (LQTS) and 1 case each of Brugada syndrome and an anomalous coronary artery.23 Fourteen of 15 (93%) serious conditions were identified by ECG, and only one identified because of prior symptoms.23 In another study of 2491 athletes undergoing preparticipation screening, application of the ESC, Seattle and ‘refined’ criteria led to abnormal ECG rates of 22.3%, 11.6% and 5.3%, respectively, all with 100% sensitivity for the pathological conditions detected.24
Effective use of ECG in the cardiovascular care of athletes requires that abnormal findings receive appropriate secondary investigations to confirm or exclude conditions associated with SCD. However, the clinical response to abnormal ECG findings may vary based on physician training and experience. This document provides recommendations for the evaluation of specific ECG abnormalities.
Limitations
While ECG increases the ability to detect underlying cardiovascular conditions that place athletes at increased risk of SCD, ECG as a diagnostic tool has limitations in both sensitivity and specificity. Specifically, ECG can suggest or detect cardiomyopathies, ion channelopathies, myocarditis and ventricular pre-excitation, yet other causes of SCD in young athletes such as anomalous coronary arteries, premature coronary atherosclerosis and aortopathies are not readily detected by ECG. Thus, even if correctly interpreted, an ECG will not detect all conditions predisposing to SCD, and evaluation of cardiovascular symptoms, a concerning family history or abnormal physical examination requires a more comprehensive investigation.
An objective of developing ECG standards is to improve the accuracy and reproducibility of interpretation. Indeed, studies have demonstrated that systematic evaluation of an athlete’s ECG using standardised criteria improves interpretation accuracy among physicians across disciplines.14 25 However, inter-observer variability and the reliability of ECG standards even among experienced physicians remains a major concern.26 27 In one study, paediatric cardiologists, without use of a standardised criteria set, achieved a sensitivity of only 68% and a specificity of 70% for recognition of abnormal ECG patterns that occur infrequently but represent conditions causing SCD.28
The importance of accurate electrode placement
Misplaced limb leads can cause errors in axis measurement and pseudo-Q waves. Misplaced precordial electrodes downward can cause pseudo-Q waves and failure to detect ST segment depression. Upward misplacement of the precordial leads can simulate myocardial injury, pericarditis or the Brugada type 2 pattern, due to pseudo-ST segment elevation. Right and left arm lead reversal is recognised by negative P waves, a negative QRS complex and T wave inversion (TWI) in leads I and aVL but not in the lateral precordial leads (V5-V6).
Accurate placement of the precordial leads can be challenging. Leads V1, V2 and V4 have distinct bony landmarks for their placement that are critical for the placement of V3, V5 and V6. V1 and V2 are close to the sternum in the fourth intercostal space (usually just above the nipple level). V4 is in the fifth intercostal space (usually just below the nipple line) and at the mid-clavicular line. V3 is placed on a line between V2 and V4. V5 and V6 are on a horizontal plane set by V4 and do not curve along the interspace. They intersect with vertical lines established by the anterior axillary fold (V5) and the mid-point of the axilla (V6).
Normal ECG findings in athletes
Overview of physiological cardiac adaptations to regular exercise
Regular and long-term participation in exercise (minimum of 4 hours per week) is associated with unique electrical manifestations that reflect enlarged cardiac chamber size and increased vagal tone. These ECG findings in athletes are considered normal, physiological adaptations to regular exercise and do not require further evaluation (figure 1; table 1).
Increased cardiac chamber size
Voltage criteria for left ventricular hypertrophy (LVH) are commonly met on an athlete’s ECG. These reflect a physiological increase in cardiac mass from athletic cardiac remodelling (figure 2). Likewise, voltage criteria for right ventricular hypertrophy (RVH) and incomplete right bundle branch block (RBBB) are also common ECG findings in athletes and considered a result of increased right ventricular (RV) size secondary to regular training.
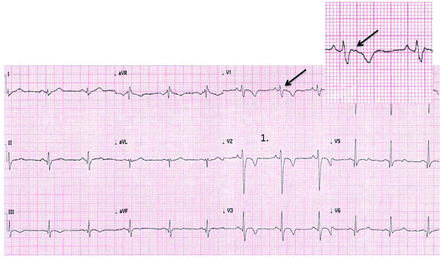
ECG of a 29-year-old male asymptomatic soccer player showing sinus bradycardia (44 bpm), early repolarisation in I, II, aVF, V4-V6 (arrows), voltage criteria for left ventricular hypertrophy (S-V1 + R-V5 >35 mm) and tall, peaked T waves (circles). These are common, training-related findings in athletes and do not require more evaluation.
Increased vagal tone
Common consequences of increased vagal tone include early repolarisation, sinus bradycardia, and sinus arrhythmia (figure 2). Other, less common markers of increased vagal tone are junctional or ectopic atrial rhythms, first-degree atrioventricular (AV) block, and Mobitz type I second-degree AV block (Wenckebach phenomenon).
QRS voltage criteria for ventricular hypertrophy
Left ventricular hypertrophy
QRS voltage can be influenced by a variety of factors. Male sex, athletic activity and younger age are associated with higher QRS voltage, while obesity and pulmonary disease may lower voltage.29 All existing ECG criteria for LVH correlate poorly with increased left ventricular (LV) wall thickness and LV mass on imaging studies.30 Although there are several voltage criteria to define LVH, the Sokolow-Lyon criteria are used most commonly, defined as the sum of the S wave in V1 and the R wave in V5 or V6 (using the largest R wave) as >3.5 mV (35 small squares with a standard amplification of the ECG at 10 mm/1 mV). Trained athletes often satisfy QRS voltage criteria for LVH, with up to 64% of athletes fulfilling the Sokolow-Lyon index (figure 2).31–36
The presence of isolated QRS voltage criteria for LVH does not correlate with pathology in athletes and is present in isolation (without other associated ECG abnormalities) in fewer than 2% of patients with HCM.30–32 37–40 Although clearing athletes with isolated voltage criteria for LVH may not detect a very small minority of individuals with HCM, such individuals have a milder phenotype with a low arrhythmogenic risk.40 Conversely, pathological LVH is commonly associated with additional ECG features such as TWI in the inferior and lateral leads, ST segment depression and pathological Q waves (figure 3).23 41 Therefore, the isolated presence of high QRS voltages that fulfil voltage criteria for LVH in the absence of other ECG or clinical markers suggestive of pathology are considered part of the normal and training-related ECG changes in athletes related to physiological increases in cardiac chamber size and/or wall thickness and does not in itself require further evaluation. However, the additional presence of TWI, ST segment depression or pathological Q waves should raise the possibility of pathological LVH and should prompt further evaluation.
ECG from a patient with HCM demonstrating QRS voltage criteria for LVH in association with deep TWI and ST segment depression predominantly in the lateral leads (I, aVL, V4–V6), voltage criteria for left atrial and right atrial enlargement and left axis deviation. HCM, hypertrophic cardiomyopathy; LVH, left ventricular hypertrophy; TWI, T wave inversion.
Right ventricular hypertrophy
Voltage criteria for RVH are also fairly common in athletes with up to 13% of athletes fulfilling the Sokolow-Lyon index (R wave in V1 + largest S wave in V5 or V6 >10.5 mV).21 33 The presence of QRS voltage criteria for RVH correlates poorly with increased RV wall thickness on echocardiography.21 Most importantly, QRS voltages for RVH, when present in isolation, do not correlate with underlying pathology in athletes, in particular arrhythmogenic right ventricular cardiomyopathy (ARVC) or pulmonary hypertension.21
In a study comparing 627 athletes to patients with established ARVC or pulmonary hypertension, none of the athletes with isolated RVH by voltage criteria had any RV pathology on advanced cardiac imaging, and none of the patients with ARVC or pulmonary hypertension exhibited voltage criteria for RVH without additional ECG abnormalities.21 Based on these considerations, it is reasonable to conclude that, similar to voltage criteria for LVH, isolated QRS voltage for RVH is part of the normal spectrum of ECG findings in athletes and in the absence of other ECG or clinical markers of pathology does not require further evaluation.
Incomplete right bundle branch block
Incomplete RBBB is defined by a QRS duration <120 ms with a RBBB pattern: terminal R wave in lead V1 (commonly characterised as an rSR’ pattern) and wide terminal S wave in leads I and V6 (figure 4). Studies suggest that the mildly delayed RV conduction in athletes is caused by RV remodelling, with increased cavity size and resultant increased conduction time, rather than an intrinsic delay within the His-Purkinje system.42 Therefore, incomplete RBBB represents a phenotype of cardiac adaptation to exercise and in the absence of other features suggestive of disease does not require further evaluation.
ECG demonstrates incomplete RBBB with rSR’ pattern in V1 and QRS duration of <120 ms. Incomplete RBBB is a common and normal finding in athletes and does not require additional evaluation. RBBB, right bundle branch block.
Early repolarisation
By convention, early repolarisation is defined as elevation of the QRS-ST junction (J-point) by ≥0.1 mV often associated with a late QRS slurring or notching (J wave) affecting the inferior and/or lateral leads (figure 2).43–45 Early repolarisation is a common finding in healthy populations (2%–44%) and is more prevalent in athletes, young individuals, males and black ethnicity.43 46–50 Early repolarisation consisting of J-point elevation with concave ST segment elevation and a peaked T wave is present in up to 45% of Caucasian athletes and 63%–91% of black athletes of African-Caribbean descent (hereto referred to as ‘black athletes’).32 33
Some studies on survivors of cardiac arrest and patients with primary ventricular fibrillation (VF) have suggested an association between early repolarisation and the risk of VF.44 51 A study of middle-aged individuals demonstrated the presence of an early repolarisation pattern in the inferior leads accompanied by a horizontal or descending ST segment after the J-point, was associated with an increased risk of arrhythmic death, while a rapidly ascending ST segment, the dominant ST pattern in healthy athletes, was associated with a benign prognosis.43 46 Early repolarisation of some subtypes appears to be a dynamic process in athletes which is affected directly by exercise training, increasing in frequency at times of peak fitness.48 49 52 Although further studies are warranted to fully elucidate the mechanisms and prognostic implications of early repolarisation in competitive athletes, to date there are no data to support an association between inferior early repolarisation and SCD in athletes. Based on current evidence, all patterns of early repolarisation, when present in isolation and without clinical markers of pathology, should be considered benign variants in athletes. 53
Repolarisation findings in black athletes
The ECG expression of the athlete’s heart is influenced by several factors. Over the past decade, ethnicity has emerged as a major determinant of cardiac adaptation to exercise with black athletes exhibiting a higher prevalence of ECG anomalies, including repolarisation changes. Notably, more than two-thirds of black athletes exhibit ST segment elevation and up to 25% demonstrate TWI on their ECG.23 33 54 55
Black athletes also commonly demonstrate a repolarisation variant consisting of J-point elevation and convex ST segment elevation in the anterior leads (V1-V4) followed by TWI (figure 5). A study of 904 black male athletes of African descent demonstrated that 13% exhibited isolated TWI in leads V1-V4 compared with only 4% of black sedentary controls.33 The majority of anterior TWI were preceded by J-point elevation and convex (domed) ST segment elevation. None of the athletes with anterior TWI showed symptoms or signs of cardiomyopathy despite comprehensive evaluation and a 5-year follow-up period.33 Similar findings have been described in female and adolescent black athletes.54–56 Based on these considerations, TWI in leads V1-V4 when preceded by J-point elevation and convex ST segment elevation should be considered part of the ‘black athlete’s heart’ and should not result in further investigation, in the absence of other clinical or ECG features of cardiomyopathy.
ECG from a black athlete demonstrating voltage criteria for LVH, J-point elevation and convex (‘domed’) ST segment elevation followed by TWI in V1-V4 (circles). This is a normal repolarisation pattern in black athletes. LVH, left ventricular hypertrophy; TWI, T wave inversion.
Considerations in athletes aged 12–16 years: the ‘juvenile’ ECG pattern
TWI confined to the anterior precordial leads may be considered a normal age-related pattern in adolescent athletes up to the age of 16 years. The term ‘juvenile’ ECG pattern is used to denote TWI or a biphasic T wave beyond lead V2 in adolescents who have not reached physical maturity (figure 6). The juvenile pattern was present in 10%–15% of white, adolescent athletes aged 12 years but only in 2.5% of white athletes aged 14–15 years.32 57 58 Anterior TWI that extends beyond lead V2 is rare (0.1%) in white athletes aged ≥16 years or younger athletes who had completed puberty when assessed by a paediatrician.32 57 Based on current evidence, TWI in the anterior leads (V1-V3) in adolescent athletes <16 years of age (or prepubertal athletes) should not prompt further evaluation in the absence of symptoms, signs or a family history of cardiac disease.
Normal and abnormal patterns of TWI. (A) Anterior TWI in V1-V3 in a 12-year-old asymptomatic athlete without a family history of SCD considered a normal ‘juvenile’ pattern. (B) TWI in V1-V4 in a 17-year-old asymptomatic mixed race (Middle-Eastern/black) athlete without a family history of SCD. This is a normal repolarisation pattern in black athletes. (C) Biphasic TWI in V3 in a 31-year-old asymptomatic black athlete without a family history of SCD. Anterior biphasic T waves are considered normal in adolescents <16 years old and in adults when found in a single lead, most commonly V3. (D) Abnormal TWI in V1-V6 in an adult symptomatic former soccer player with genetically confirmed ARVC and a positive family history of SCD (brother died at 26 years of age). (E) An ECG demonstrating the type 1 Brugada pattern with high take-off ST elevation ≥2 mm with downsloping ST segment elevation followed by a negative symmetric T wave in V1-V2. (F) Inferolateral TWI in leads I, II, III, aVF, V2-V6 and ST segment depression in leads II, aVF, V4-V6 in a 31-year-old asymptomatic professional soccer referee. These markedly abnormal findings require a comprehensive evaluation to exclude cardiomyopathy. ARVC, arrhythmogenic right ventricular cardiomyopathy; SCD, sudden cardiac death; TWI, T wave inversion.
Sinus bradycardia
Sinus bradycardia is defined as a heart rate <60 beats per minute (bpm) and is present in up to 80% of highly trained athletes.32 35 In normal sinus rhythm, the heart rate is determined by the balance between the sympathetic and parasympathetic nervous systems. Resting sinus bradycardia is particularly prevalent in endurance athletes due to increased vagal tone and possible structural atrial remodelling.59 60 In the absence of symptoms such as fatigue, dizziness, or syncope, heart rates ≥30 bpm are considered normal in highly trained athletes. Sinus bradycardia should resolve with the onset of physical activity.
Sinus arrhythmia
Sinus arrhythmia, the physiological fluctuation in heart rate with breathing, is considered a normal finding and should not be confused with sinus node dysfunction or sick sinus syndrome. Differentiating features that suggest sinus node dysfunction include:
lack of rhythmic changes in the heart rate,
abrupt sustained rate increases and decreases,
prolonged pauses or periods of sinus arrest,
inappropriate rate response to exercise (including slowed acceleration and an inappropriately rapid deceleration),
any association with clinical symptoms such as exercise intolerance, pre-syncope and syncope.
In sinus arrhythmia, the P wave axis remains normal in the frontal plane and the fluctuation in heart rate should resolve with the onset of exercise.
Junctional escape rhythm
A junctional escape (nodal) rhythm occurs when the QRS rate is faster than the resting P wave or sinus rate, which is typically slower in athletes due to increased vagal tone (figure 7). The R-R interval is regular in a junctional escape rhythm, and the QRS complexes are narrow unless the baseline QRS has a bundle branch block. Sinus rhythm should resume with the onset of physical activity.
A 28-year-old asymptomatic Caucasian handball player demonstrating a junctional escape rhythm (red arrows). Note the constant RR interval between beats.
Ectopic atrial rhythm
In an ectopic atrial rhythm, P waves are present but of different morphology compared with the sinus P wave, typically with a rate ≤100 bpm. Ectopic P waves are most easily seen when the P waves are negative in the inferior leads (figure 8). Occasionally, two different P wave morphologies may be seen and this is known as a ‘wandering atrial pacemaker’. A junctional escape rhythm or wandering atrial pacemaker is observed in up to 8% of all athletes under resting conditions.32 Ectopic atrial rhythms occur due to a slowed resting sinus rate from increased vagal tone in athletes. Sinus rhythm should resume with the onset of physical activity.
ECG shows an ectopic atrial rhythm. The atrial rate is 63 beats per minute and the P wave morphology is negative in leads II, III and aVF (arrows), signifying an ectopic atrial rhythm.
First-degree AV block
First-degree AV block is found in up to 7.5% of athletes on a resting ECG and is characterised by a prolonged (>200 ms) PR interval.32 35 61 This represents a delay in AV nodal conduction in athletes, due to increased vagal activity or intrinsic AV node changes, and typically resolves with the onset of exercise.
Mobitz type I (Wenckebach) second-degree AV block
In Mobitz type I second-degree AV block, the PR interval progressively lengthens from beat to beat, until there is a non-conducted P wave with no QRS complex (figure 9). The first PR interval after the dropped beat is shorter than the last conducted PR interval before the dropped beat. This represents a greater disturbance of AV nodal conduction than first-degree AV block, but is usually a normal finding in asymptomatic, well-trained athletes, and 1:1 conduction should return with the onset of exercise.
ECG shows Mobitz type I (Wenckebach) second-degree AV block demonstrated by progressively longer PR intervals until there is a non-conducted P-wave (arrows) and no QRS complex. Note the first PR interval after the dropped beat is shorter than the last conducted PR interval prior to the dropped beat.
Sport-specific ECG considerations
Adaptive changes in cardiac structure, function and electrophysiology occur in response to regular physical training. A strong relationship exists between the amount of training performed, resulting fitness, and extent of adaptive cardiac remodelling. Using measured VO2 max as a marker of athlete fitness, a robust linear association has been demonstrated between fitness and cardiac size.62 In this context, it is not surprising that many ECG features associated with athletic training are more prevalent and more profound in athletes exposed to the greatest training stimulus. This concept has most frequently been addressed by comparing athletes involved in team, skill-based or strength sports with those competing in endurance exercise. In a study comparing the ECGs of 1010 elite non-endurance and 251 endurance athletes, the latter group demonstrated more frequent bradycardia, voltage criteria for LVH and early repolarisation—all features considered common, benign and training-related.63 However, uncommon features such as TWI extending to lead V3 was more common (4.0 vs 1.1%, p<0.0001) in endurance athletes as compared with non-endurance athletes.63 Elite rowers also demonstrate a higher prevalence of TWI in the anterior septal leads compared with non-endurance or mixed athletic cohorts.64 In addition, the prevalence of incomplete and complete RBBB appears to increase with cardiac size, thereby suggesting these findings are more frequently observed in endurance athletes.42
Borderline ECG findings in athletes
Overview of borderline ECG findings in athletes
Recent data suggests that some ECG findings previously categorised as abnormal may also represent normal variants or the result of physiological cardiac remodelling in athletes and do not usually represent pathological cardiac disease. These ECG findings, specifically axis deviation, voltage criteria for atrial enlargement and complete RBBB, have been categorised as ‘borderline’ findings in athletes (figure 1; table 1).
Axis deviation and voltage criteria for atrial enlargement
Several studies have shown that the Seattle criteria reduces the number of positive ECGs compared with the 2010 ESC criteria by at least 40% and improves specificity without compromising sensitivity.18 23 24 65 Despite the improvements, more recent publications suggest that axis deviation and voltage criteria for atrial enlargement account for a large number of ECG patterns that are classified as abnormal in athletes but do not correlate with cardiac pathology. In a large study of 2533 athletes aged 14–35 years and 9997 controls of similar age, isolated left or right axis deviation and isolated left or right atrial enlargement comprised 42.6% of all ECG findings.22 The athletes revealed a slightly higher prevalence of these findings compared with controls (5.5% vs 4.4%; p=0.023). Specifically, athletes showed a higher prevalence of left axis deviation and left atrial enlargement versus controls (1.46% vs 0.96%; p=0.028% and 2.13% vs 1.37%; p=0.007, respectively), particularly in those who trained over 20 hours per week.22 In contrast, there were no significant differences in the prevalence of right axis deviation and right atrial enlargement between the groups (1.11% vs 1.10%; p=0.983% and 0.83% vs 0.92%; p=0.664, respectively).22
Athletes with left axis deviation or left atrial enlargement exhibited larger left atrial and ventricular dimensions compared with athletes with a normal ECG and those with other physiological ECG changes consistent with athlete’s heart.22 However, there were no appreciable differences in the number of athletes with cardiac dimensions exceeding predicted upper limits between the two groups. In contrast, there were no differences in cardiac dimensions between athletes with right axis deviation or right atrial enlargement compared with athletes with normal or usual physiological ECG changes.22 Echocardiographic evaluation of the 579 athletes and controls with isolated axis deviation or voltage criteria for atrial enlargement failed to identify any major structural or functional abnormalities, and the prevalence of minor congenital abnormalities was similar in these individuals to those with normal ECGs.22
Exclusion of axis deviation or atrial enlargement reduced the false-positive rate from 13% to 7.5% and improved specificity from 90% to 94% with a minimal reduction in sensitivity (91% to 89.5%)%).22 A US study of 508 university athletes also revealed that of the 49 athletes considered to have abnormal ECGs at least 29 (59%) exhibited either voltage criteria for left atrial enlargement alone or in combination with large QRS complexes, and subsequent echocardiography revealed a structurally normal heart or findings consistent with athletic training.39
These findings suggest that right axis deviation and right atrial enlargement occurring in isolation or in association with other electrical markers of ‘athlete’s heart’ are probably normal variants, whereas left axis deviation and left atrial enlargement may reflect a relative increase in LV dimensions in some athletes.
Complete RBBB
Although incomplete RBBB is common in young athletes, the significance of complete RBBB is less certain. This particular ECG pattern was placed in the abnormal category in the 2010 ESC recommendations and more recent ‘refined’ criteria, although considered a normal finding in the Seattle criteria if the QRS duration was <140 ms.5 10 23 Complete RBBB is detected in approximately 1% of the general population and large datasets in young adult athletes reveal a prevalence of 0.5%–2.5%.12 66–68 In a study of 510 US collegiate athletes, RBBB was reported in 13 (2.5%) athletes and compared with athletes with normal QRS complexes and athletes with incomplete RBBB.42 The athletes with complete RBBB exhibited larger right ventricular dimensions and a lower right ventricular ejection fraction but preserved fractional area change. None of the athletes with complete RBBB or incomplete RBBB had pathological structural cardiac disease suggesting that that this particular ECG pattern may be a manifestation of more extreme right ventricular adaptation to exercise. These patterns among trained athletes could represent a spectrum of structural and physiological cardiac remodelling characterised by RV dilation with resultant QRS prolongation and a relative reduction in the RV systolic function at rest.42
Based on the aforementioned considerations, left axis deviation, left atrial enlargement, right axis deviation, right atrial enlargement and complete RBBB are considered borderline variants in athletes. The presence of any one of these findings in isolation or with other recognised physiological electrical patterns of athletic training does not warrant further assessment in asymptomatic athletes without a family history of premature cardiac disease or SCD. Conversely, the presence of more than one of these borderline findings in combination places the athlete in the abnormal category warranting additional investigation (figure 10).
ECG from an asymptomatic 22-year-old black male athlete demonstrating complete right bundle branch block (QRS ≥120 ms), left axis deviation (−57°) and right atrial enlargement (P wave ≥2.5 mm in II and aVF). The presence of two or more borderline ECG findings warrants additional investigation to exclude pathological cardiac disease.
Abnormal ECG findings in athletes
Overview of ECG criteria for the detection of pathological cardiac disorders in athletes
Many pathological cardiac disorders associated with SCD exhibit abnormalities on a resting ECG. TWI is the most consistent electrical abnormality in patients with cardiomyopathy. ST segment depression, pathological Q waves, and left bundle branch block (LBBB) are also recognised in cardiomyopathic disorders and ischaemic heart disease. Primary electrical disease such as ventricular pre-excitation, LQTS and Brugada syndrome are suggested and/or diagnosed by abnormal ECG findings. None of these abnormal findings as defined in this section are recognised features of athletic training and always require further assessment to exclude the presence of intrinsic cardiac disease (figure 1, table 1, table 2). When ECG abnormalities are identified, an athlete’s personal and family history should be thoroughly questioned as part of a comprehensive clinical investigation.
Abnormal T wave inversion
T waves represent repolarisation or recovery of the ventricular myocardium and constitute the final waveform of the cardiac cycle on ECG. T waves can be described by numerous attributes including duration, symmetry, skewness, slope of the ascending and descending waveform, and most importantly, directionality. Directionality (ie, positivity or negativity) refers to the direction of T wave deflection with regards to the electrically neutral ECG baseline, conventionally defined as the PR segment. T wave directionality is typically concomitant with the net vector of the QRS complex and is thus deflected above the baseline (ie, positive) in most leads. Normal exceptions, leads in which T waves are routinely negative, include aVR, III and V1. T waves deflected negatively are referred to as TWI. When present in leads aside from aVR, III and V1, TWI may have an association with underlying structural heart disease. TWI ≥1 mm in depth in two or more contiguous leads (excluding leads aVR, III and V1) in an anterior, lateral, inferolateral or inferior territory is abnormal (with the exception of TWI confined to leads V1-V4 in black athletes and leads V1-V3 in all athletes aged <16 years) and should prompt further evaluation for underlying structural heart disease (table 1; table 2).
Clinical considerations
The relationship between abnormal TWI and several forms of structural heart disease including HCM, ARVC, dilated cardiomyopathy (DCM), left ventricular non-compaction and myocarditis is well documented. In a cohort of young (age <35 years) asymptomatic patients with confirmed HCM, 62% exhibited abnormal TWI.69 In a similar study of asymptomatic or mildly symptomatic patients with HCM, the prevalence of abnormal TWI was reported at 80%.70 Most recently, the prevalence of abnormal TWI among athletes with newly diagnosed HCM was reported in excess of 90%.71 In a study comparing 1124 athletes with 255 patients with HCM, TWI in V4-V6 were present in 38% of patients with HCM versus 0.8% of athletes (p<0.001) (figure 3).72
ARVC is similarly associated with abnormal TWI. Specifically, the presence of TWI in the right precordial leads (V1-V3) or beyond in the absence of a complete RBBB is common among patients with ARVC and has been adopted as a major criterion for diagnosis (figure 11).73 74 Recently, the deleterious prognostic implications of TWI involving non-anterior lead territories among patients with ARVC, particularly inferior lead TWI, have been demonstrated.75 76
ECG from a 30-year-old patient with ARVC showing anterior TWI in V1-V3 preceded by a flat or downsloping ST segment without J-point elevation. PVCs are also present. ARVC, arrhythmogenic right ventricular cardiomyopathy; PVC, premature ventricular contraction.
Abnormal TWI is relatively infrequent among healthy white competitive athletes with a reported prevalence ranging from <1.0% to 4% based on factors including age, sex and type of sport but is reported in as many as 12% of black athletes.18 32 33 39 56 64 77–79 Based on the overlap with the cardiomyopathies, abnormal TWI in asymptomatic athletes warrants a comprehensive clinical assessment to exclude underlying cardiomyopathy. There are no data relating to the significance of flat or biphasic T waves in athletes. Similar to TWI, this panel would recommend further evaluation of biphasic T waves where the negative portion is ≥1 mm in depth in ≥2 leads.
Evaluation
Lateral or inferolateral TWI
There is mounting evidence that TWI in the lateral or inferolateral leads, in any athlete, are associated with the presence of quiescent cardiomyopathy.33 79–81 Recommendations for the evaluation of abnormal TWI and other clinical considerations are presented in table 2.
Although rarely seen in athletes without subsequently identified structural heart disease, TWI affecting the lateral leads (V5-V6, I and aVL) is considered abnormal and should prompt comprehensive investigation irrespective of ethnicity, including cardiac MRI, when echocardiography is non-diagnostic. Echocardiographic quality is variable and may have limited ability to detect hypertrophy of the anterolateral LV wall and apex.82 Contrast-enhanced cardiac MRI provides superior assessment of myocardial hypertrophy and may demonstrate late gadolinium enhancement which is a non-specific marker suggesting myocardial fibrosis. Exercise ECG testing and Holter monitoring also should be considered in the evaluation of lateral or inferolateral TWI, especially for ‘grey zone’ hypertrophy (maximal LV wall thickness 13–16 mm) without late gadolinium enhancement, where the diagnosis of HCM remains uncertain. In such cases, the presence of ventricular tachycardia during exercise or Holter may support a pathological diagnosis and is useful in risk stratification.83
Cardiac MRI should be a standard component of the assessment for markedly abnormal ECGs suggestive of apical HCM, specifically ECGs with deep (>−0.2 mV) TWI and ST segment depression in the lateral or inferolateral leads in which echocardiography may not provide adequate assessment of the LV apex and inferior septum. If cardiac MRI is not available, echocardiography with contrast should be considered as an alternative investigation for apical HCM. For athletes with lateral TWI, regular follow-up with serial cardiac imaging is necessary even when the initial evaluation is normal, in order to monitor for the development of a cardiomyopathy phenotype (figure 12).80 81
(A) ECG from an 18-year-old black basketball player demonstrating abnormal TWI extending into V5. Initial cardiac imaging was non-diagnostic. (B) ECG from the same athlete at age 20 showing abnormal TWI in the inferolateral leads with the development of deep TWI and ST segment depression in V4-V6. Follow-up cardiac MRI demonstrated distinct findings of apical HCM with a maximum left ventricular wall thickness of 21 mm with small foci of late gadolinium enhancement. Athletes with TWI in V5 and/or V6 require serial evaluation for the development of cardiomyopathy. HCM, hypertrophic cardiomyopathy; TWI, T wave inversion.
Anterior TWI
Anterior TWI is a normal variant in asymptomatic adolescent athletes age <16 years and in black athletes when preceded by J-point elevation and convex ST segment elevation.84 Anterior TWI involving lead V3 is also reported in white, adult, predominantly endurance athletes.63 However, anterior TWI in leads V1-V2/V3 also is a recognised pattern in patients with ARVC and more rarely HCM (figure 11). There are discrepancies among existing guidelines relating to the extent of anterior TWI inversion before considering further investigations.5 6 16 23
A large study of over 14 000 white adults aged 16–35 years, including over 2500 athletes showed that anterior TWI had a prevalence of 2.3%.85 Anterior TWI was more common in females and athletes and was confined to leads V1-V2 in almost all individuals, and only exceeded beyond V2 in 1% of females and 0.2% of males.85 None of the individuals with anterior TWI were diagnosed with a cardiomyopathy following comprehensive investigation indicating that this particular ECG pattern is non-specific in low-risk populations. Based on this report, it would be justifiable to investigate only athletes with anterior TWI beyond V2 in the absence of other clinical or electrical features of ARVC.
Specific information about the J-point and preceding ST segment may help differentiate between physiological adaptation and cardiomyopathy in athletes with anterior TWI affecting leads V3 and/or V4. A recent study comparing anterior TWI in a series of black and white healthy athletes, patients with HCM and patients with ARVC showed that in athletes with anterior TWI, the combination of J-point elevation ≥1 mm and TWI confined to leads V1-V4 excluded either cardiomyopathy with 100% negative predictive value, regardless of ethnicity.84 Conversely, anterior TWI associated with minimal or absent J-point elevation (<1 mm) could reflect a cardiomyopathy.84 These data require duplication in larger studies but may prove useful in the assessment of a small proportion of white endurance athletes who exhibit anterior TWI and in athletes of black/mixed ethnicity.34
In most non-black athletes age ≥16 years, anterior TWI beyond lead V2 should prompt further evaluation given the potential overlap with ARVC. The extent of evaluation is dependent on the specific ECG findings suggestive of ARVC and will be more extensive in the presence of warning symptoms or significant family history. In athletes age ≥16 years with TWI beyond V2, concurrent findings of J-point elevation, ST segment elevation or biphasic T waves more likely represent athlete’s heart, while the absence of J-point elevation or a coexistent depressed ST segment is more concerning for ARVC.84 Other ECG findings suggestive of ARVC in the presence of anterior TWI include low limb lead voltages, prolonged S wave upstroke, ventricular ectopy with LBBB morphology and epsilon waves.74 A combination of tests is needed to effectively make the diagnosis of ARVC and may include echocardiography, cardiac MRI, Holter monitoring, exercise ECG test and signal averaged ECG.
Repolarisation variants in the anterior precordial leads in black athletes must be distinguished from pathological repolarisation changes found in ARVC (figure 13). In ARVC, the J-point and/or ST segment is usually isoelectric or depressed prior to TWI, in contrast to the J-point elevation and ‘domed’ ST segment elevation which is the hallmark feature of the physiological repolarisation variant in black athletes.
Examples of physiological (A) and pathological T wave inversion (TWI) (B). Panel (A) demonstrates TWI in V1-V4 preceded by J-point elevations and convex ‘domed’ ST segment elevation (green circles). This should not be confused with pathological TWI as seen in panel (B) which demonstrates TWI in V1-V6 with absent J-point elevation and a downsloping ST segment (red circles).
Inferior TWI
The significance of TWI confined to the inferior leads (II, III and aVF) has not been studied in detail. TWI isolated only to the inferior leads is not a strong predictor of cardiomyopathy in the absence of other abnormal ECG or clinical features. However, this finding cannot be attributed to physiological remodelling and thus warrants further investigation with, at minimum, an echocardiogram until larger studies with longer follow-up prove otherwise. Cardiac MRI should be considered based on the echocardiographic findings or clinical suspicion. Serial assessment, on an annual basis, is advised until data from longitudinal studies become available.
ST segment depression
While ST segment depression is common among patients with cardiomyopathy, it is not a feature of athletic training (figure 3). Prevalence estimates of ST segment depression among patients with HCM range from 60%–70%, and in one study the territorial distribution of ST segment depression among patients with HCM was associated with the risk of sudden death or appropriate implantable cardioverter-defibrillator therapy.41 86 87 In contrast, ST segment depressions are extremely rare in young athletes with structurally normal and physiologically remodelled hearts.33 55 68 72 ST segment depression (relative to the isoelectric PR segment) in excess of 0.05 mV (0.5 mm) in two or more leads should be considered an abnormal finding requiring definitive evaluation for underlying structural heart disease.
Evaluation
Echocardiography is the minimum evaluation for athletes with ST segment depression to investigate for underlying cardiomyopathy. Cardiac MRI should be considered based on the echocardiographic findings or clinical suspicion.
Pathological Q waves
Q waves are defined as any initial negative deflection of the QRS complex and can be found with both physiological electrical activation of the ventricle and with certain pathological conditions, including cardiomyopathy, myocardial infarction and conduction abnormalities.
Several pathological disorders can lead to the development of exaggerated (deep or wide) Q waves or unexpected Q waves in atypical leads. HCM commonly results in asymmetric septal hypertrophy which can produce abnormal Q waves due to increased septal forces, septal fibrosis and asymmetric electrical activation (figure 14). Indeed, pathological Q waves are among the most common abnormal ECG findings in HCM and present in 32%–42% of patients.41 69 Pathological Q waves can also result from prior transmural myocardial infarction. Any loss of myocardium and/or loss of myocardial electrical activity due to infarction, infiltration or fibrosis may lead to unopposed electrical activation of the opposite segments. Additionally, early activation from a bypass tract such as in WPW distorts the location of initial ventricular activation and may result in broad or atypical Q waves.
ECG from an 18-year-old female swimmer demonstrating deep and wide pathological Q waves in V4-V6, I and aVL. Diagnostic testing revealed hypertrophic cardiomyopathy.
Pathological Q waves also may be a result of lead misplacement. In particular, a pseudo-septal infarct pattern with pathological Q waves in leads V1-V2 is commonly due to high lead placement relative to cardiac position. This finding is more common in women than men, and in one series 64% of subjects lost this QS pattern on repeat ECG.88
A physiological increase in myocardial mass, such as LVH from athletic remodelling, also can result in increased Q wave voltage, and the change in cardiac geometry and electrical axis may distort the location of Q waves slightly. Thus, although Q waves can be a marker of underlying heart conditions, differentiating physiological from pathological Q waves can be challenging.
Pathological Q waves have been reported in approximately 1%–2% of all athletes, and may be higher in males and black athletes.23 89 For asymptomatic athletes, pathological Q waves were previously defined as >3 mm in depth or >40 ms in duration in two or more leads (except III and aVR).6 10 In practice, however, this criterion is a common source of false-positive ECG results as trained athletes with physiological LVH and thin adolescent athletes may have increased precordial voltages and deep lateral or inferior Q waves. Long narrow Q waves (>3 mm in depth) as previously defined by the Seattle criteria have been shown to be an unreliable marker for pathological LVH in athletes with increased QRS voltage. In a report of 13 335 adolescents age 14–18 years screened with ECG, none of the 206 (1.5%) individuals with abnormal Q waves but no other ECG abnormality had pathological findings on limited echocardiogram consistent with cardiomyopathy.90 Thus, modification of the criteria for pathological Q waves has the potential to greatly improve the specificity of ECG interpretation.
The use of a Q/R ratio overcomes some of these issues by normalising Q wave depth to the degree of proceeding R wave voltage. Case–control analyses of athletes and patients with HCM suggest that this will decrease the false-positive rate without compromising sensitivity for the detection of cardiomyopathy.23 24 Therefore, the consensus of this panel based on existing scientific data is to modify the definition for pathological Q waves in athletes as a Q/R ratio ≥0.25 or ≥40 ms in duration in two or more contiguous leads (except III and aVR).
Evaluation
An ECG with abnormal Q waves should be carefully examined for the possibility of a bypass tract, looking for a short PR interval or evidence of a delta wave. If the pathological Q waves are isolated to leads V1-V2, the ECG should be repeated, including re-placing the ECG leads to ensure proper positioning. Persistence of a QS pattern should undergo additional evaluation.
Pathological Q waves in two or more contiguous leads warrants further investigation with echocardiography to exclude cardiomyopathy. A detailed family history and assessment of risk factors for coronary artery disease also should occur, especially in athletes age ≥30 years as stress testing may be warranted in athletes with suspicion of prior myocardial infarction or multiple risk factors for coronary artery disease.
If the echocardiogram is normal and there are no other concerning clinical findings or ECG abnormalities, no additional testing is generally necessary. If pathological Q waves are present along with other ECG abnormalities, such as ST segment depression or TWI, or if suspicious clinical findings are present, additional evaluation with cardiac MRI should be considered.
Complete LBBB
LBBB is found in less than 1 in 1000 athletes but is common in patients with cardiomyopathy and ischaemic heart disease.9 41 72 91 92 Thus, complete LBBB always should be considered an abnormal finding and requires a comprehensive evaluation to rule out a pathological cardiac disorder.
LBBB is recognised by a QRS ≥120 ms, predominantly negative QRS complex in lead V1 (QS or rS), and upright notched or slurred R wave in leads I and V6 (figure 15). Q waves are often absent in leads I, V5 and V6, and the ST segment and T waves are usually in the opposite direction than the QRS.93
ECG with complete LBBB demonstrating a QRS ≥120 ms, predominantly negative QRS complex in lead V1, upright R wave in leads I and V6, and ST segments and T waves in the opposite direction of the QRS. LBBB is always an abnormal finding in athletes and warrants a comprehensive evaluation to exclude myocardial disease. LBBB, left bundle branch block.
Evaluation
Athletes with complete LBBB require a thorough investigation for myocardial disease including echocardiography and a cardiac MRI with perfusion study.
Profound non-specific intraventricular conduction delay
Epidemiological studies of non-specific intraventricular conduction delay in the general population have shown an increased risk of cardiovascular death and have been documented among patients with cardiomyopathy.94 95 The significance of non-specific intraventricular conduction delay with normal QRS morphology in healthy, asymptomatic athletes is uncertain.96 The physiology underlying intraventricular conduction delay in athletes remains incompletely understood but likely includes some combination of neurally mediated conduction fibre slowing and increased myocardial mass. In patients with LVH, left ventricular mass seems to be closely related to QRS duration.97
While the exact cut-off to trigger more investigation in athletes with a non-specific intraventricular conduction delay remains unclear, this panel recommends that marked non-specific intraventricular conduction delay ≥140 ms in athletes, regardless of QRS morphology, is abnormal and should prompt further evaluation. In asymptomatic athletes with isolated non-specific intraventricular conduction delay <140 ms, no further diagnostic evaluation is required. Athletes with cardiovascular symptoms or a concerning family history of sudden death or suspected cardiomyopathy with a non-specific intraventricular conduction delay <140 ms or in tandem with other ECG abnormalities should be evaluated further.
Evaluation
In asymptomatic athletes with profound non-specific intraventricular conduction delay, an echocardiogram is recommended to evaluate for myocardial disease. Other testing may be indicated depending on echocardiographic findings or clinical suspicion.
Epsilon waves
Epsilon waves are defined as distinct low-amplitude signals localised between the end of the QRS complex and onset of the T wave in leads V1-V3 (figure 16). Epsilon waves are challenging to detect and appear as a small positive deflection or notch just beyond the QRS in leads V1-V3. The presence of epsilon waves is a highly specific ECG marker and represents a major diagnostic criterion for ARVC.74
ECG in a young athlete with arrhythmogenic right ventricular cardiomyopathy showing several abnormal features including anterior T wave inversion (V1–V4) preceded by a non-elevated J-point and ST segment, an epsilon wave in V1 (magnified and marked with arrow), delayed S wave upstroke in V2, and low voltage (<5 mm) QRS complexes in limb leads I and aVL.
High interobserver variability has been found in the assessment of epsilon waves.98 However, epsilon waves are typically a manifestation of more advanced disease and unlikely to be an isolated ECG finding.98 In patients with ARVC that express an epsilon wave, 89% also manifest TWI in the right precordial leads and 100% have a delayed S wave upstroke (prolonged terminal activation duration) ≥55 ms from the nadir of the S wave to the end of the QRS complex.98
Evaluation
Evaluation of epsilon waves, especially in combination with right precordial TWI or delayed S wave upstroke, requires the exclusion of possible ARVC through a combination of tests including echocardiography, cardiac MRI, Holter monitoring, exercise ECG test and signal averaged ECG.
Ventricular Pre-excitation
Ventricular pre-excitation occurs when an accessory pathway bypasses the AV node resulting in abnormal conduction to the ventricle (pre-excitation) with shortening of the PR interval and widening of the QRS. This is evident on the ECG as the Wolf-Parkinson-White (WPW) pattern defined as a PR interval <120 ms, the presence of a delta wave (slurring of the initial QRS) and a QRS duration >120 ms (figure 17).93 The WPW pattern occurs in approximately 1/1000 to 4/1000 athletes.9 12 66 99 The presence of an accessory pathway can predispose an athlete to sudden death because rapid conduction of atrial fibrillation across the accessory pathway can result in VF.
ECG demonstrating the classic findings of Wolf-Parkinson-White pattern with a short PR interval (<120 ms), delta wave (slurred QRS upstroke) and prolonged QRS (>120 ms).
Evaluation
A short PR interval associated with a delta wave is consistent with the WPW pattern and warrants further assessment of the refractory period of the accessory pathway. A short PR interval in isolation without a widened QRS or delta wave in an asymptomatic athlete should not be considered for further assessment.
Asymptomatic athletes with WPW pattern should be investigated for the presence of a low-risk or high-risk accessory pathway. Non-invasive risk stratification begins with an exercise stress test in which abrupt, complete loss of pre-excitation at higher heart rates suggests a low-risk accessory pathway.100 101 Intermittent pre-excitation during sinus rhythm on a resting ECG is also consistent with a low-risk pathway and may obviate the need for an exercise test.102
If non-invasive testing cannot confirm a low-risk pathway or is inconclusive, electrophysiology testing should be considered to determine the shortest pre-excited RR interval during atrial fibrillation.100 If the shortest pre-excited RR interval is ≤250 ms (240 bpm), then the accessory pathway is deemed high risk.100 103 Young athletes with a shortest pre-excited RR interval of ≤250 ms should proceed with transcatheter pathway ablation.100 An echocardiogram also should be considered due to the association of WPW with Ebstein’s anomaly and cardiomyopathy. Some physicians may choose to subject all competitive athletes with WPW pattern involved in moderate-intensity or high-intensity sport to electrophysiological studies irrespective of the results of the exercise test or 24-hour ECG on the premise that high catecholamine concentrations during very intensive exercise may modify the refractory period of an accessory pathway in a fashion that cannot be reproduced during laboratory tests.
Prolonged QT interval
Congenital LQTS is a potentially lethal, genetically mediated ventricular arrhythmia syndrome with the hallmark ECG feature of QT prolongation. Symptoms if present include arrhythmic syncope, seizures or aborted cardiac arrest/sudden death stemming from torsades de pointes and VF. The pathophysiology of LQTS involves delayed ventricular repolarisation originating primarily from loss-of-function mutations in genes encoding voltage gated potassium channels that govern phase 3 repolarisation. LQTS is estimated to affect 1 in 2000 individuals, and this may be underestimated given the subpopulation of so-called ‘normal QT interval’ or ‘concealed’ LQTS.104
Autopsy negative sudden unexplained death represents 25%–40% of sudden unexpected deaths in persons under age 40 years.3 105–107 In cases that lack necropsy findings to establish a cause of death, cardiac ion channelopathies have been implicated by post-mortem genetic testing as the probable cause in up to 25%–35% of sudden unexplained death in selected cohorts.108–111
Calculating the QTc
Accurate measurement and manual confirmation of the computer derived QT interval corrected for heart rate (QTc) is critical as the accuracy of computer generated QTc values is about 90%–95%. Studies have suggested the ability of cardiologists and even heart rhythm specialists to accurately measure the QTc is suboptimal.112 However, accurate assessment of the QTc can be achieved by adhering to the following six principles113:
Most ECG devices use the Bazett’s heart rate correction formula (QTc = QT/√RR; note the RR interval is measured in seconds).114 Although there are many heart rate correction formulas for the QTc, it is recommended to use Bazett’s correction to confirm the computer’s QTc as population-based QTc distributions most frequently have used Bazett-derived QTc values.
Bazett’s formula loses accuracy at slow and fast heart rates; underestimating the inherent QTc at heart rates <50 bpm, and overestimating the QTc at heart rates >90 bpm. Accordingly, if an athlete has a heart rate <50 bpm, a repeat ECG after mild aerobic activity is recommended to achieve a heart rate closer to 60 bpm where the formula is most accurate. Likewise, for heart rates >90 bpm, repeating the ECG after additional resting time and ensuring the patient is not unduly cold, hot or anxious may help achieve a lower heart rate where Bazett’s formula will be more accurate.
If sinus arrhythmia is present with beat to beat variation in heart rate, an average QT interval and average RR interval should be used to improve accuracy. Importantly, taking the maximum QT interval and dividing it by the square root of the shortest RR interval on the ECG will grossly overestimate the QTc.115
To properly perform a manual QT measurement, it is critical to identify the end of the T wave since the onset of the QRS is typically seen easily. Leads II and V5 usually provide the best delineation of the T wave, and these leads are often used for the rhythm strip at the bottom of the ECG.
Low amplitude U waves, common in the anterior precordial leads, should not be included in the QT calculation. Such U wave inclusion will greatly exaggerate the QTc. Instead, the ‘Teach-the-Tangent’ or ‘Avoid-the-Tail’ method to delineate the end of the T wave should be followed (figure 18).113
The morphology of the T wave, not just the length of the QT interval, also can suggest the presence of LQTS.116 For instance, a notched T wave in the lateral precordial leads where the amplitude of the second portion of the T wave following the notch is greater than the first portion of the T wave may represent LQT-2 even in the absence of overt QT prolongation.
This figure illustrates the ‘Teach-the-Tangent’ or ‘Avoid-the-Tail’ method for manual measurement of the QT interval. A straight line is drawn on the downslope of the T wave to the point of intersection with the isoelectric line. The U wave is not included in the measurement.
With this framework, the easiest and most efficient way to confirm the computer-derived QTc is to examine lead II and/or V5 and determine if the manually measured QT interval matches the computer’s QT measurement. If there is concordance within about 10 ms of each other, one can trust that the computer can derive accurately an average RR interval and complete the Bazett’s calculation. If, however, the manually measured QT interval is >10 ms different than the computer’s QT measurement, an average RR interval should be determined and the QTc recalculated using the Bazett’s formula.
QTc cut-offs
Given the overlap between QTc distributions in population-derived cohorts of healthy individuals compared with patients with genetically confirmed LQTS, it must be acknowledged that no screening programme will identify all persons with LQTS.117–120 Instead, the QTc cut-off value, where the QTc measurement compels further evaluation, must be chosen carefully to balance the frequency of abnormal results and the positive predictive value that LQTS has been detected incidentally.
Published definitions of a ‘prolonged QTc’ requiring further evaluation have varied. Prior guidelines from the ESC for ECG interpretation in athletes define a QTc of >440 ms in males and >460 ms in females (but <500 ms) as a ‘grey zone’ requiring further evaluation, and a QTc ≥500 ms, otherwise unexplained and regardless of family history and symptoms, as indicative of unequivocal LQTS.5 In the USA, the American Heart Association/American College of Cardiology/Heart Rhythm Society guideline has dropped the term ‘borderline’ QT prolongation and instead annotates a QTc >450 ms in men and >460 ms in women as ‘prolonged QTc’.121 Concern has been raised that these QTc cut-offs will produce a high number of false-positive test results if followed in a screening population of athletes.117 More recent consensus statements on ECG interpretation in athletes have recommended that male athletes with a QTc >470 ms and female athletes with a QTc >480 ms undergo further evaluation for LQTS to better balance false-positive and false-negative findings.6 10 These cut-off values are around the 99th percentile and consistent with thresholds defined by the 36th Bethesda Conference.122 This consensus group also recommends QTc values of >470 ms in males and >480 ms in females to define the threshold of QT prolongation that warrants further assessment in asymptomatic athletes.
Short QT interval
While an abnormal cut-off for a short QT interval has been included in past ECG interpretation guidelines, the precise cut-off and clinical significance of a short QT interval in athletes is unknown. Data from over 18 000 asymptomatic young British individuals suggests that the prevalence of a QTc <320 ms is 0.1%; suggesting an abnormal cut-off value of <320 ms is pragmatic.123 However, over a mean follow-up period of 5.3 years, none of the individuals with a short QT <320 ms experienced any adverse events, syncope or sudden death.123 Based on the rarity of this finding and absence of data to suggest long-term morbidity in asymptomatic athletes, the consensus of this panel recommends that a short QT interval only be investigated in the context of concerning clinical markers such as syncope, premature atrial fibrillation, ventricular arrhythmias or a relevant family history.
Evaluation
An athlete identified with a prolonged QTc (≥470 ms in males;≥480 ms in females) should undergo further evaluation. It is critical that an athlete with a single QTc reading above these threshold values not be obligated a diagnosis of LQTS, but rather that these cut-off values have triggered the need for additional evaluation. The importance of additional evaluation but not a premature diagnosis of LQTS was demonstrated in a study of 2000 elite athletes in which 7 (0.4%) had a prolonged QTc (range 460–570 ms).124 A QTc of <500 ms in the absence of symptoms or familial disease was unlikely to represent LQTS. In contrast, a QTc ≥500 ms was highly suggestive of LQTS as all three athletes with a QTc value of >500 ms exhibited one of paradoxical prolongation of the QTc during exercise, a confirmatory genetic mutation or prolonged QTc in a first-degree relative.124 As outlined below, personal symptoms, family history, scoring systems, ECG features, stress ECG and genetic testing may be needed to clarify the diagnosis.
A personal history of exercise/emotion/auditory-triggered syncope or seizures and a family history of exertional syncope, exercise/auditory-triggered ‘epilepsy’, postpartum-timed syncope/seizure, unexplained motor vehicle accidents, unexplained drowning and premature, unexplained sudden death <50 years of age should be reviewed. If the personal/family history is positive, the athlete should be referred to a heart rhythm specialist for further evaluation. If the personal/family history is negative, a repeat ECG should be obtained (ideally on a different day). If the follow-up ECG is below the QTc cut-off values, then no additional evaluation is needed and the athlete should be reassured.
On the other hand, if the repeat ECG still exceeds the QTc cut-off values, then a screening ECG of the athlete’s first-degree relatives (parents and siblings) should be considered and the athlete should be referred to a heart rhythm specialist or cardiologist for the possibility of newly discovered LQTS. Reversible, extrinsic factors, such as electrolyte abnormalities (hypokalemia) or the presence of QT prolonging medications, must also be evaluated. If an athlete’s ECG shows a QTc >500 ms and no reversible causes are identified, then the athlete should be referred immediately to a heart rhythm specialist or cardiologist as the probability of LQTS and future adverse events has increased.125 Further testing, such as provocative treadmill stress testing, epinephrine QT stress testing and genetic testing need to be considered carefully and should be performed and interpreted by a cardiologist familiar with the disease.
LQTS is diagnosed based on a combination of symptoms, family history, ECG findings and genetic testing, and the Schwartz-Moss score used to invoke low, intermediate and high probability.126–128 Genetic testing for LQTS is recommended for any athlete where a cardiologist has an index of suspicion for LQTS (intermediate or high probability score), or for an asymptomatic patient with no family history but an incidental ECG finding with a QTc >480 ms pre-puberty and >500 ms post-puberty that is confirmed on repeat ECG testing.129
Brugada type 1 pattern
Brugada syndrome is a primary electrical disease characterised by the distinctive Brugada ECG pattern of ‘high take-off’ ST segment elevation in the right precordial leads (figure 6). Although three types were described, only the type 1 Brugada pattern is now considered diagnostic.130–132 Brugada syndrome predisposes to ventricular tachyarrhythmias in the absence of clinically demonstrable structural heart disease, and sudden death in patients with Brugada syndrome occurs more often during states of enhanced vagal tone.133 Loss of function mutations in the sodium channel gene SCN5A accounts for up to 20% of cases.130 134 Brugada syndrome is estimated to cause up to 4% of all sudden deaths in the general population and 5%–20% of sudden unexplained deaths with a structurally normal heart at autopsy.130 135
The type 1 Brugada pattern consists of a coved rSr’ pattern, ST-segment elevation ≥2 mm, and inversion of the terminal portion of the T wave in leads V1, V2 and V3. Classic type 2 and 3 Brugada patterns have a ‘saddleback’ appearance with J-point elevation ≥2 mm, ST segment elevation >1 mm in type 2 and ≤1 mm in type 3, and either a positive or biphasic T wave. A recent consensus document grouped the classic types 2 and 3 as type 2.131
Distinguishing between true type 1 or 2 patterns and a host of Brugada-like ECG patterns can be challenging. Brugada-like patterns may be caused by both physiological (normal variant, early repolarisation, incomplete RBBB) and pathological (ARVC, pulmonary hypertension, hyperkalemia) conditions.131 136 Pharmacological and ECG manoeuvres may clarify the diagnosis. Confirmation of proper precordial lead placement is paramount, as high placement of the V1 and V2 electrodes in the second and third intercostal space (rather than the fourth intercostal space) can accentuate a type 1 Brugada ECG pattern in known Brugada patients but also produce type 2 like patterns in athletes.137
The coved ST segment elevation in type 1 Brugada pattern results in a broad r’ and should be distinguishable from the upsloping ST segment elevation of early repolarisation in an athlete. In this regard, the ‘Corrado index’ measures the ST elevation at the start of the ST segment/J-point (STJ) and 80 ms after the start of the ST segment (ST80).138 In type 1 Brugada pattern, the downsloping ST segment will have a STJ/ST80 ratio >1, while the initial upsloping of the ST segment found in early repolarisation patterns in an athlete will produce an STJ/ST80 ratio <1 (figure 19).
Brugada type 1 ECG (left) should be distinguished from early repolarisation with ‘convex’ ST segment elevation in a trained athlete (right). Vertical lines mark the J-point (STJ) and the point 80 ms after the J-point (ST80), where the amplitudes of the ST segment elevation are calculated. The ‘downsloping’ ST segment elevation in Brugada pattern is characterised by a STJ/ST80 ratio >1. Early repolarisation patterns in an athlete show an initial ‘upsloping’ ST segment elevation with STJ/ST80 ratio <1.
Evaluation
The type 1 Brugada ECG pattern is not a recognised variant of athlete’s heart and should raise the possibility of a sodium ion channelopathy. Patients with a type 1 ECG pattern should be referred to a cardiac electrophysiologist for further evaluation, regardless of symptoms. If the pattern is unclear, confirm correct lead placement, repeat the ECG if necessary, and perform a high precordial lead ECG with V1 and V2 placed in the second or third intercostal space. If the type 1 pattern is seen on high precordial lead ECG, then referral to a heart rhythm specialist is indicated. Consideration should be given to potential accentuating factors for a Brugada-like ECG pattern, such as hyperkalemia, fever, medications with sodium ion channel blocking properties and lead placement.
The type 2 Brugada ECG pattern may overlap with repolarisation changes in the anterior leads in black athletes and endurance athletes. Multiple criteria for evaluating type 2 patterns have been proposed; however, in the absence of symptoms or a concerning family history, athletes with type 2 patterns do not require further testing.
Profound sinus bradycardia
Sinus bradycardia is a hallmark feature of athletic conditioning with heart rates commonly between 40–60 bpm or even slower. A resting heart rate ≤30 bpm or a sinus pause ≥3 s may be normal in a well-trained athlete but nevertheless should prompt further evaluation.
Evaluation
Evaluation of profound sinus bradycardia should include assessing the chronotropic response to mild aerobic activity, such as running on the spot or climbing stairs. If the heart rate increases appropriately and the athlete is asymptomatic, no further testing is necessary. If there is no change in heart rate or in athletes with a history of symptoms such as presyncope or syncope, further testing should be performed to exclude primary sinus node disease. Exercise testing is useful in this situation to provide an objective measure of the heart rate response to aerobic activity.
Profound first-degree heart block
Mild to moderate first-degree heart block with a PR interval of 200 to 399 ms may be present in athletes due to increased vagal tone. A PR interval ≥400 ms is significantly prolonged and requires further evaluation.
Evaluation
A small amount of aerobic activity should be performed to assess if the PR interval shortens appropriately. If the PR interval normalises with exercise, the PR prolongation is due to vagal mechanisms and is benign. If the PR interval does not shorten, or there are symptoms of syncope or a family history of cardiac disease or sudden death, further evaluation should be performed.139 An exercise test can demonstrate more clearly if the PR interval changes at higher heart rates. Depending on the clinical scenario, an echocardiogram or ambulatory ECG monitor may be indicated.
High-grade AV block
Mobitz type II second-degree AV block and third-degree (complete) AV block are pathological disruptions in AV conduction and abnormal findings in athletes. In Mobitz type II AV block, there are intermittently non-conducted P waves with a fixed PR interval. In complete AV block, there are more P waves than QRS complexes, and the ventricular rhythm is regular due to an undisturbed junctional or ventricular pacemaker. Complete heart block can be confused with AV dissociation without block; a situation where the junctional pacemaker is faster than the sinus node, leading to more QRS complexes than P waves. Intermittent ventricular capture by sinus P waves (resulting in an irregular ventricular response) excludes complete AV block. AV dissociation without block is the expression of autonomic mismatch between AV and sinus nodal modulation, but is not pathological. Like all other functional disturbances, a small exercise load with repeat ECG recording will show resolution of the ECG findings in AV dissociation. Complete heart block requires further evaluation for underlying cardiac disease.
Evaluation
If Mobitz II AV block or complete AV block is detected, further evaluation includes an echocardiogram, ambulatory ECG monitor, and exercise ECG test. Based on these results, laboratory testing and cardiac MRI may be considered. Referral to a heart rhythm specialist is essential.
Multiple premature ventricular contractions
Multiple (≥2) premature ventricular contractions (PVCs) are uncommon and present in <1% of athlete ECGs (figure 19).9 12 When 2 or more PVCs are recorded on a standard (10 s) ECG, it is possible the athlete has a high 24 hours PVC burden. While multiple PVCs are most likely benign in a highly trained athlete, their presence may be the hallmark of underlying heart disease.140 In athletes with ≥2000 PVCs per 24 hours, up to 30% were found to have underlying structural heart disease, in contrast to 3% and 0% in those with <2000 and <100 PVCs per day, respectively.141Over half of the athletes with ≥2000 PVCs also had bursts of non-sustained ventricular tachycardia.141 Therefore, the finding of ≥2 PVCs on an ECG should prompt more extensive evaluation to exclude underlying structural heart disease.
Evaluation
The extent of evaluation for ≥2 PVCs is controversial and excluding pathology may be difficult. At a minimum, an ambulatory Holter monitor, echocardiogram and exercise stress test should be performed. The availability of modern small, leadless ambulatory recorders allows for longer ECG monitoring, including during training and competition, to exclude complex ventricular arrhythmias. If the Holter and echocardiogram are normal and the PVCs suppress with exercise, no further evaluation is recommended for an asymptomatic athlete. However, in athletes with ≥2000 PVCs per 24 hours or with episodes of non-sustained ventricular tachycardia, or with an increasing burden of ectopy during an incremental exercise test, additional evaluation may include contrast-enhanced cardiac MRI and more invasive EP study.142 Therefore, many such cases require referral to a specialist centre.143 Although some studies have suggested that regression of the PVC burden with detraining indicates a good prognosis, other studies have not confirmed this.144–146 Thus, detraining as a diagnostic or therapeutic measure is not recommended.
Considerations in high-dynamic athletes
In high-level endurance athletes, such as cyclists, triathlon athletes and rowers, concern has been raised about the high volume and pressure loads on the right ventricle that could promote or induce ARVC. Competitive sport definitively accelerates and enhances the phenotypic expression of desmosomal mutations causing ARVC.147 148 There also is evolving evidence that high-dynamic exercise sustained over a long period of time may result in ‘exercise-induced’ ARVC, even in the absence of desmosomal mutations or a familial history.143 149–151 PVCs originating from the right ventricular outflow tract conduct with LBBB and an inferior axis and are usually regarded as benign when associated with an otherwise normal ECG; however, this PVC morphology can also be present in patients with early ARVC particularly when the QRS exceeds 160 ms.152 In contrast, PVCs originating from the main body of the right ventricle typically show a LBBB pattern and superior axis (predominantly negative QRS vector in V1 and the inferior leads) and may be associated with right ventricular pathology particularly in the context of other ECG abnormalities (figure 20). Therefore, a lower threshold for extensive evaluation of a single PVC may be warranted in high-dynamic athletes, especially when ≥25 years of age and with a LBBB morphology and superior axis.
ECG from a patient with arrhythmogenic right ventricular cardiomyopathy. Note multiple premature ventricular contractions with a left bundle branch block pattern and superior axis (negative QRS vector in inferior leads).
Atrial tachyarrhythmias
Atrial tachyarrhythmias, comprised of supraventricular tachycardia (SVT), atrial fibrillation and atrial flutter, are heart rhythms >100 bpm which originate in the sinus node, atrial tissue or AV node. Sinus tachycardia is the most common atrial tachyarrhythmia but is very rarely due to intrinsic cardiac disease.
SVT, atrial fibrillation and atrial flutter are rarely seen on a resting ECG in athletes and require investigation. One study demonstrated just 6 of 32 561 young athletes during ECG screening with an atrial tachyarrhythmia, four (0.01%) with ectopic atrial tachycardia and two (<0.01%) with atrial fibrillation.9 An examination of ECGs from the South Korean Army also found zero soldiers with an atrial tachyarrhythmia.153 Atrial tachyarrhythmias are rarely life-threatening and usually lead to symptoms like palpitations, shortness of breath, chest pressure, dizziness, neck pounding or syncope from rapid heart rates. AV dyssynchrony also may limit peak physical activity. More importantly, they can be associated with other conditions that can lead to SCD, including LQTS, WPW, Brugada syndrome, myocarditis, congenital heart disease and any form of cardiomyopathy.
Evaluation
For paroxysmal SVT, a repeat ECG when not in SVT should be obtained if possible. If the Valsalva manoeuvre, carotid sinus massage or the diving reflex is used to terminate the arrhythmia, a rhythm strip should be obtained to assess whether the SVT terminates with a P wave or QRS complex which can help elucidate the mechanism of the SVT. An echocardiogram, ambulatory ECG monitor and exercise treadmill test should be completed, and referral to a heart rhythm specialist may be indicated for consideration of electrophysiology study and ablation.
If atrial fibrillation or flutter is found, an echocardiogram should be completed to assess for structural heart disease and anti-coagulation considered based on standard guidelines.154 An ambulatory ECG monitor should be used to assess if the rhythm is paroxysmal or persistent and what the ventricular rate is throughout the day. An exercise treadmill test also should be completed to assess the maximum heart rate in atrial fibrillation. A thorough family history may elucidate an underlying genetic cause. Depending on what these results show, cardiac MRI, electrophysiology study with possible ablation and/or genetic testing may be considered.
If resting sinus tachycardia >120 bpm is seen, a repeat ECG should be considered after a period of rest as recent exercise or anxiety may be the cause. Other underlying aetiologies may be sought, including fever, infection, dehydration, stimulant use, anaemia, hyperthyroidism or, rarely, underlying cardiac or pulmonary disease.
Ventricular arrhythmias
Ventricular couplets, triplets and non-sustained ventricular tachycardia always require investigation. Alone, these are not life-threatening arrhythmias, but can be a marker for underlying cardiac pathology or lead to sustained ventricular tachycardia which may cause SCD. These ventricular arrhythmias may be idiopathic or secondary to the cardiomyopathies, ion channelopathies or other diseases such as myocarditis, myocardial infarction or sarcoidosis.
Evaluation
If ventricular arrhythmias are seen, the evaluation should include a thorough family history, an echocardiogram to evaluate for structural heart disease, cardiac MRI to assess for ARVC or other cardiomyopathies, ambulatory ECG monitor and exercise ECG test. Depending on these results, further evaluation may be needed including electrophysiology study or genetic testing.
Considerations in athletes ≥30 years of age
In athletes ≥30 years of age, coronary artery disease is the most common cause of SCD.106 107 The number of older athletes at all levels of competition is increasing worldwide. In addition, older athletes may be less fit compared with 20–30 years ago, increasing the possibility of underlying coronary artery disease.155 In a cohort study of Swedish competitive cross-country runners with a mean age of 60 years, 2% were shown to have severe underlying cardiovascular disease, mainly coronary artery disease.156 While the overall health benefits of regular exercise and physical activity are unequivocal, exercise may be a trigger for acute cardiac events in individuals with underlying and silent coronary artery disease. The risk of an acute cardiovascular event during exercise seems to be U-shaped, with the greatest risk conferred in those who are physically inactive but also in those exercising at the highest intensity. Therefore, it is important to consider relevant changes on a resting ECG that may represent occult coronary artery disease in asymptomatic athletes ≥30 years of age.
While resting ECGs have a low sensitivity for coronary artery disease, some ECG patterns may suggest underlying coronary artery disease such as TWI, pathological Q waves, ST segment depression, left or right bundle branch block, abnormal R wave progression, left anterior hemiblock and atrial fibrillation.157–159 Thus, the major role of a resting ECG in screening for coronary artery disease may be in older athletes generally considered to be at an intermediate risk, where an abnormal ECG would place them into a high-risk group warranting further testing.158 160 161
Evaluation
Additional evaluation for underlying coronary artery disease should be considered in asymptomatic older athletes with TWI, pathological Q waves, ST segment depression, left or right bundle branch block, abnormal R wave progression, left anterior hemiblock and atrial fibrillation. Initial testing should normally include an exercise stress test, resting echocardiogram and assessment of traditional risk factors for coronary artery disease. When indicated, this evaluation may be complemented by coronary CT angiography or a functional stress test.
ECG patterns that require serial follow-up
All abnormal ECG findings discussed in this document should prompt a comprehensive evaluation for underlying structural and/or electrical heart disease. The specific components of this evaluation should be individualised to the athlete based on the presence or absence of symptoms and relevant family history and on the specific attributes of the abnormal ECG. While the abnormal findings delineated in this document were chosen to maximise specificity for true underlying disease and therefore minimise the likelihood of false-positive ECG testing, no single ECG abnormality has perfect accuracy. Further, ECG abnormalities may precede the development of overt structural heart disease in athletes with a genetic predisposition to cardiomyopathy.41 80 81 Athletes who demonstrate one or more overtly abnormal ECG findings may therefore undergo comprehensive clinical evaluations that reveal no definitive evidence of true pathology. In these cases, the clinical significance of the presenting abnormal ECG remains uncertain.
Several common heritable cardiomyopathies including HCM, ARVC and familial DCM may present with ECG abnormalities prior to the onset of overt heart muscle pathology. The natural history of athletes with markedly abnormal ECGs but structurally normal hearts on initial evaluation was first documented in a study of 81 athletes in which 6% (5/81) developed overt cardiomyopathy (HCM, n=3; ARVC, n=1; DCM, n=1) during an average follow-up period of 9 years.80 In a more recent series of athletes with abnormal ECG findings, 6% (5/85) with initially unrevealing clinical evaluations developed features consistent with cardiomyopathy (HCM, n=2; ARVC, n=1; arrhythmic event, n=2) during a 12-month follow-up period.81
Therefore, athletes with abnormal ECGs suggestive of cardiomyopathy and initially normal clinical evaluations should be followed with serial evaluation during and after their competitive athletic careers. Specifically, asymptomatic athletes with abnormal TWI, ST segment depression, pathological Q waves, LBBB and profound intraventricular conduction delay (QRS duration ≥140 ms) require serial cardiac imaging to rule out the development of a cardiomyopathic disease (figure 12). These athletes may be permitted to participate in competitive athletics without restriction contingent on longitudinal follow-up. Follow-up assessments should include a medical history to ascertain new cardiovascular symptoms or otherwise unexplained decrements in exercise tolerance, a physical examination and non-invasive cardiac imaging as deemed suitable for the appropriate suspected form of disease. These evaluations should be conducted on an annual basis while participating in competitive athletics or more frequently as determined on an individual basis. Athletes should be educated about the importance of longitudinal surveillance to promote ownership of the process if they change teams, medical staff or locations.
Temporary restriction during secondary evaluation of ECG abnormalities
Temporary restriction from athletic activity should be considered for athletes with abnormal ECGs of uncertain clinical significance until secondary investigations are completed. While the absolute risk of suffering an adverse cardiac event due to underlying structural or electrical heart disease while awaiting definitive evaluation is low, the risk is not negligible. Although this conservative approach is accepted commonly for an athlete with symptoms, the asymptomatic athlete who has an abnormal ECG during pre-participation screening presents other challenges.
Athletes in this situation often have limited time to participate in team-based tryouts, and sport restriction may jeopardise their athletic eligibility or generate pressure from coaches, managers, parents or the athletes themselves.162 To circumvent some of these challenges, proper advanced planning prior to pre-participation screening is required to establish avenues for prompt secondary testing and consultation for ECG abnormalities. If ECG is included in the cardiovascular screening of athletes, it must be conducted with adequate cardiology oversight and resources to assist with the secondary investigation of ECG abnormalities. Ideally, pre-participation screening should be performed at a time point that permits athletes to undergo additional cardiovascular testing prior to the onset of organised competitive athletics. The optimal duration of time between pre-participation screening and the beginning of organised team based training will vary as a function of local resources.
Considerations in the symptomatic athlete and in the athlete with a family history of sudden death or hereditary cardiovascular disease
Symptoms suggestive of underlying cardiovascular disease including exertional chest discomfort, inappropriate exertional dyspnoea, unexplained/unheralded syncope, unexplained seizure-like activity, and exertional palpitations, particularly when coupled with abnormal ECG findings, are strongly suggestive of a pathological process. In the symptomatic athlete either with or without an abnormal ECG, comprehensive testing should be tailored to the presenting symptoms and performed in a timely fashion. Asymptomatic athletes with a family history of sudden death or a heritable cardiomyopathy or ion channelopathy may similarly carry an increased risk of true occult disease.163 164 Great care must be taken to confirm the nature of the family history as athletes may have incomplete or erroneous information about their relatives. In the evaluation of an athlete with a compelling family history, genetic testing permits a more definitive assessment of individual and familial risk for specific disease processes such as HCM, ARVC and congenital LQTS. The use of genetic testing and its interpretation should be performed by a cardiovascular specialist with expertise in clinical genetics.
Psychological considerations of caring for the athlete with potentially lethal cardiac disease
Athletes diagnosed with serious cardiac diseases, regardless of the method used for disease detection, are at risk for psychological morbidity and represent an emotionally vulnerable population.165 Risk factors for increased psychological morbidity include a higher level of competition, complete disqualification from athletic competition, daily reminders of disease (ie, medication or implantable cardioverter defibrillator) and unanticipated outcomes (ie, failed cardiac procedure).
Informing an athlete that he/she has an underlying cardiac disorder that may affect sports participation is difficult. In fact, many athletes may not completely comprehend the nature of their disease, and several follow-up discussions are likely necessary. Assessment of emotional well-being should be a standard part of all follow-up evaluations, and psychological support for the athlete may be enhanced by a multi-disciplinary approach. In cases where competitive sport is not recommended, additional measures should consider helping the athlete refocus away from athletics, such as excelling in school or becoming involved in other team-related activities (ie, coaching). Athletes disqualified from competitive athletics should be offered exercise recommendations thought to be safer to foster long-term physical and mental health. While exercise guidelines for individuals with inherited cardiac disorders exist, more research is needed to fully understand safe, disease-specific physical activity recommendations.166 167
Conclusion
Accurate ECG interpretation in athletes requires adequate training and an attention to detail to distinguish physiological ECG findings from abnormal ECG findings that might indicate the presence of cardiac pathology. Cardiac adaptation and remodelling from regular athletic training produce common ECG alterations that could be mistaken as abnormal. Whether performed for screening or diagnostic purposes, it is critical that physicians responsible for the cardiovascular care of athletes be guided by ECG interpretation standards that improve disease detection and limit false-positive results. The international consensus standards presented on ECG interpretation and the evaluation of ECG abnormalities serve as an important foundation for improving the quality of cardiovascular care of athletes. As new scientific data become available, revision of these recommendations may be necessary to further advance the accuracy of ECG interpretation in the athletic population.
Acknowledgments
The 2nd Summit on Electrocardiographic Interpretation in Athletes was sponsored by the American Medical Society for Sports Medicine, the FIFA Medical Assessment and Research Center (F-MARC), and the National Collegiate Athletic Association (NCAA). Participating medical societies included the American College of Cardiology (ACC) Sports & Exercise Council, and the Section on Sports Cardiology of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR), a registered branch of the European Society of Cardiology (ESC).
This statement has been endorsed by the following societies: American Medical Society for Sports Medicine, Austrian Society of Sports Medicine and Prevention, Brazilian Society of Cardiology - Department of Exercise and Rehabilitation, British Association for Sports and Exercise Medicine, Canadian Academy of Sport and Exercise Medicine, European College of Sports and Exercise Physicians, ESC Section of Sports Cardiology, FIFA, German Society of Sports Medicine and Prevention, IOC, Norwegian Association of Sports Medicine and Physical Activity, South African Sports Medicine Association, Spanish Society of Cardiology Sports Cardiology Group, Sports Doctors Australia, and the Swedish Society of Exercise and Sports Medicine. The American College of Cardiology (ACC) affirms the value of this document: ACC supports the general principles in the document and believes it is of general benefit to its membership.
References
Footnotes
This statement has been endorsed by the following societies: American Medical Society for Sports Medicine (AMSSM), Austrian Society of Sports Medicine and Prevention, Brazilian Society of Cardiology - Department of Exercise and Rehabilitation (SBC - DERC), British Association for Sports and Exercise Medicine (BASEM), Canadian Academy of Sport and Exercise Medicine (CASEM), European College of Sports and Exercise Physicians (ECOSEP), European Society of Cardiology (ESC) Section of Sports Cardiology, Fédération Internationale de Football Association (FIFA), German Society of Sports Medicine and Prevention, International Olympic Committee (IOC), Norwegian Association of Sports Medicine and Physical Activity (NIMF), South African Sports Medicine Association (SASMA), Spanish Society of Cardiology (SEC) Sports Cardiology Group, Sports Doctors Australia, and the Swedish Society of Exercise and Sports Medicine (SFAIM). The American College of Cardiology (ACC) affirms the value of this document (ACC supports the general principles in the document and believes it is of general benefit to its membership).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.