Article Text
Abstract
Objective To examine the effects of short-term, medium-term and long-term resistance exercise training (RET) on measures of cardiometabolic health in adults.
Design Intervention systematic review.
Data sources MEDLINE and Cochrane Library databases were searched from inception to February 2018. The search strategy included the following keywords: resistance exercise, strength training and randomised controlled trial.
Eligibility criteria for selecting studies Randomised controlled trials published in English comparing RET≥2 weeks in duration with a non-exercising control or usual care group. Participants were non-athletic and aged ≥18 years.
Results A total of 173 trials were included. Medium-term and long-term RET reduced systolic blood pressure (−4.02 (95% CI −5.92 to −2.11) mm Hg, p<0.0001 and −5.08 (−10.04 to –0.13) mm Hg, p=0.04, respectively) and diastolic blood pressure (−1.73 (−2.88 to –0.57) mm Hg, p=0.003 and −4.93 (−8.58 to –1.28) mm Hg, p=0.008, respectively) versus control. Medium-term RET elicited reductions in fasted insulin and insulin resistance (−0.59 (−0.97 to –0.21) µU/mL, p=0.002 and −1.22 (−2.29 to –0.15) µU/mL, p=0.02, respectively). The effects were greater in those with elevated cardiometabolic risk or disease compared with younger healthy adults. The quality of evidence was low or very low for all outcomes. There was limited evidence of adverse events.
Conclusions RET may be effective for inducing improvements in cardio metabolic health outcomes in healthy adults and those with an adverse cardio metabolic risk profile.
PROSPERO registration number CRD42016037946.
- cardiovascular
- exercise training
- systematic review
- strength training
Statistics from Altmetric.com
Introduction
Cardiovascular disease is a substantial human and economic burden, responsible for 17.7 million deaths globally in 2015.1 The positive impact of regular moderate to vigorous intensity aerobic exercise (eg, brisk walking, jogging, cycling) on cardiometabolic health, including improvements in cardiopulmonary exercise capacity, blood pressure, glycaemic control, hypercholesterolaemia and vascular endothelial function,2 3 is well documented and recognised in current UK and global physical activity recommendations.4 5 However, while the health benefits of regular resistance exercise training (RET) in relation to maintaining skeletal muscle size and strength are also recognised in current physical activity recommendations, the role of RET in enhancing cardiometabolic health is less well defined.
RET is characterised by muscular activities working against an external load and may be easier than aerobic exercise to implement and sustain in the home environment as it offers an alternative way to exercise for adults who have limited space or access to equipment and time availability.6–8 Most studies of RET have focused on changes in skeletal muscle size and strength, with few investigating cardiometabolic health effects as primary outcomes although several have reported cardiometabolic variables as secondary outcomes.6 9 10
There is preliminary evidence that RET may positively alter blood lipid profile, body composition, systolic blood pressure,11–13 circulating inflammatory markers and cardiopulmonary exercise capacity.2 14 15 RET may also generate longer-lasting improvements in body fat, fasted insulin, lipid profile and systolic blood pressure than aerobic exercise.16 17 Finally, RET may have an important role in attenuating age-related physiological changes such as increases in systolic blood pressure and arterial stiffness, and the reduction of skeletal muscle mass (with associated changes in systemic physiology).3 18
Aside from the lack of RET intervention studies with a primary focus on cardiometabolic health outcomes, interpreting the impact of RET on cardiometabolic health is constrained by heterogeneity of methodology, including the duration of interventions and populations. High-quality systematic reviews and meta-analyses can help to overcome these challenges, while accounting for bias and heterogeneity, by providing more precise estimates of effect size changes. The aim of this systematic review was to assess the effects of short-term, medium-term and long-term RET programmes compared with control or usual care on cardiometabolic health outcomes in adults.
Methods
The Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines were followed19 when conducting and reporting this prospectively registered systematic review (PROSPERO ID CRD42016037946).
Eligibility criteria
We included randomised controlled trials (RCTs) published in English that compared any RET programme alone with a non-exercising control or usual care group. Participants must have been aged ≥18 years, non-athletic20 and recruited to a RET programme (eg, elastic resistance band, weight machines, etc) of at least 2 weeks’ duration, irrespective of intensity or frequency that was conducted in any setting (eg, home, hospital). We included studies where isometric RET with whole-body vibration was used. We excluded studies where RET interventions were combined with other lifestyle components or exercise modes (eg, aerobic exercise, diet, etc) to isolate the effects of RET. Studies that included at least one of the following cardiometabolic health outcomes or clinical end-points were eligible: cardiopulmonary exercise capacity (V̇O2max); flow-mediated dilatation; C reactive protein; total cholesterol; high-density lipoprotein cholesterol; low-density lipoprotein cholesterol; triglycerides; fasted glucose; fasted insulin; insulin resistance (HOMA-IR); resting blood pressure; mean arterial pressure; resting heart rate; cardiovascular mortality; all-cause mortality; non-fatal cardiovascular end-points (eg, myocardial infarction, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty; angina or angiographically defined coronary heart disease; stroke; carotid endarterectomy; peripheral arterial disease).
Search strategy
The MEDLINE Ovid and Cochrane Library databases were searched from inception to February 2018. The search strategy keywords and MeSH terms used included progressive resistance, strength training, exercise and randomised controlled trial. Details of the full search strategy can be found in online supplementary table 1. Reference lists of all relevant systematic reviews identified were searched for additional studies. All searches were conducted by the same author (REA), with search results collated using EndNote software (Thomson Reuters, New York) and duplicates removed.
Supplementary file 1
The first 10% of titles and abstracts were screened independently by two reviewers (REA and GAT) and, due to good agreement, the remaining texts were screened by one reviewer only (REA, GAT, JMS or LL).21 Screening of full texts was performed by two independent reviewers (REA and GAT) with disagreements resolved through consensus or a third reviewer being consulted (JMS).
Data extraction
Two authors (REA and SEG) independently extracted data using Microsoft Excel. Any disagreements were resolved via consensus. When more than one publication was apparent for the same trial, data were collated (online supplementary table 2). We extracted study design, participant demographics, intervention details and means and SD for all outcomes. When necessary, published protocols and trial registries were searched for further methodological detail and risk of bias assessment. If there was insufficient information, the authors (n=40) were contacted via email. Resting blood pressure was expressed in millimetres of mercury (mm Hg), resting heart rate in beats per minute (bpm), V̇O2max relative to body mass (mL/kg/min), flow-mediated dilatation as percentage, fasted insulin in microunits per millilitre (µU/ml), C reactive protein in milligrams per litre (mg/L) and glucose, lipid profile and HOMA-IR in milligrams per decilitre (mg/dL). Adverse events were also extracted.
Supplementary file 2
Risk of bias
Risk of bias was assessed by two authors independently (REA and SEG) using the Cochrane Risk of Bias tool.22 Any disagreements were resolved through consensus. We judged risk of bias on the study level as ‘low’, ‘unclear’ or ‘high’ risk.23 We used funnel plots to assess publication bias when there were more than 10 studies contributing data for an analysis.23 24 For all outcomes, we conducted sensitivity analyses. For the sensitivity analyses, we excluded studies that were judged as being at unclear risk of bias on a majority of domains of the Cochrane tool, or where at least two domains of the Cochrane tool were judged as being at high risk of bias, and ran the meta-analysis again.
Data synthesis
Meta-analyses were undertaken using Review Manager (RevMan V.5.3; Cochrane Collaboration, Oxford, UK) when more than two studies reported on the same outcome. In the pooled analysis of studies by duration, outcome data were organised into short-term (≤6 weeks), medium-term (7–23 weeks) and long-term (≥24 weeks) arbitrary categories. Where units of measurement could not be converted, standardised mean differences were used. Data are presented as mean and 95% CIs. The I² statistic was used to quantify statistical heterogeneity as follows: 0%–40%, might not be important; 30%–60%, moderate heterogeneity; 50%–90%, substantial heterogeneity; 75%–100%, considerable heterogeneity.23 Fixed-effects model was used for analysis; however, if statistical heterogeneity was noted (I2 >40%), meta-analysis was performed using a random-effects model. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the strength of evidence. Studies were downgraded if there were issues with risk of bias, consistency, precision or directness of the outcomes. The reasons for downgrading the evidence are outlined in table 1.
Criteria for downgrading the quality of outcomes using the GRADE approach
Where multiple RET groups were compared with a single control group, data for the intervention most similar to traditional RET were used for the analysis. After the initial analysis, subgroup analyses were conducted to explore sources of heterogeneity by dividing studies into three categories: healthy young adults aged 18–40 years; healthy older adults≥41 years old; older adults≥41 years old with elevated cardiometabolic risk or established disease (defined as any elevated blood marker above normal levels or overweight, obese or hypertensive participants). Adverse events were synthesised descriptively.
Results
A total of 19 040 records were retrieved from database searches, of which 5669 records were duplicates. A further 11 696 were then eliminated following screening of titles and abstracts (figure 1). Sixty-three potentially relevant papers were identified from screening of systematic review reference lists (figure 1). After full-text screening of 1738 articles, 194 manuscripts from 173 RCTs were included in this review (online supplementary file 36). Participants were individually randomised in all included trials (ie, there were no cluster RCTs).
Supplementary file 36
Preferred Reporting Items for Systematic Review and Meta-Analyses flow diagram. RCT, randomised controlled trial.
Study characteristics
The 173 RCTs comprised 6169 participants (2840 control and 3329 RET participants), with sample sizes of 5–77 per group and 13–150 per study. One hundred studies involved healthy individuals and 73 studies involved clinical populations. All included studies were published between 1978 and February 2018. Summary details of the included trials and populations are presented in online supplementary tables 2 and 3, respectively.
RET programmes mainly used weight machines (n=90 studies; 52%), a mix of free weights, bodyweight and machine exercises (n=43 studies; 25%), elastic resistance bands (n=13 studies; 8%), circuit exercises (n=12 studies; 7%), free weights (n=10 studies; 6%), ankle/leg weights (n=2 studies; 1%), isometric hand grip (n=2 studies; 1%) and isometric exercise with whole-body vibration (n=1 study).
The majority of interventions were supervised by an exercise professional (n=105 studies; 61%). One study reported data from an unsupervised intervention, and 13 (8%) used a combination of supervised and unsupervised programmes. Fifty-four studies (31%) did not report the level of supervision.
The duration of the intervention varied from ≤6 weeks (n=13), 7–23 weeks (n=129) and ≥24 weeks (n=31). The most common frequency of training was three sessions per week (n=110), followed by two sessions per week (n=36), though some studies required participants to complete the programme in one, four or five sessions per week (n=1, n=7 and n=5, respectively). The remaining studies stipulated either two to three sessions per week (n=8), three to four sessions per week (n=1) or did not report the frequency (n=5).
In the majority of studies, control participants were instructed to continue with their habitual activity (n=115/173) or were allocated to usual care (n=15). Three studies provided lifestyle advice to the control group and discussion about physical activity levels, but no structured/supervised exercise (n=3). Forty studies did not report the requirements of the control group. The included studies did not report any clinical end-points. A summary of the quality of evidence, based on risk of bias, study design, CIs and variability in results, has been collated using the GRADE approach (table 2).
GRADE summary of findings.
Risk of bias
Figure 2 shows a summary of the risk of bias decisions made per category for the included studies. Online supplementary table 4 describes risk of bias for each study in detail.
Supplementary file 4
Risk of bias summary.
Selection bias
An acceptable method of random sequence generation (ie, computer generated) was used in 36 studies, 8 studies were judged as being at high risk of bias and the remaining 129 studies were judged as being at unclear risk due to insufficient information to determine randomisation methods. The majority of studies (n=156) did not report allocation concealment and were judged as unclear. Fourteen studies were judged as being at low risk of bias as allocation was blinded. In three studies, the researchers were not blinded to the allocation process and we judged these studies as being at high risk of bias.
Performance and detection bias
All trials were at high risk of performance bias (ie, no blinding of participants to the intervention and outcomes). Lack of investigator blinding could have influenced measures of resting blood pressure and flow-mediated dilatation but is more likely to have had an impact on the motivation provided to participants during V̇O2max tests. The majority of studies (n=144) were rated as unclear for detection bias (ie, blinding of outcome assessor) due to insufficient information provided in the studies. Two studies were at high risk of detection bias, with the remaining 27 studies at low risk.
Attrition bias
The majority (n=122) of studies were judged as being at low risk of attrition bias. A further 37 studies were rated as unclear risk due to attrition rates >20% in one of the study groups (ie, control or RET). Some studies (n=14) were rated as high risk due to high dropout rates or some participants being excluded from the analysis.
Reporting bias
The majority (n=166) of studies were rated as low risk for selective reporting bias. A further four studies were classed as unclear due to a lack of description of outcome measures and three studies rated as high risk as data for some outcomes were not reported.
Publication bias
Funnel plots were produced for all outcomes, except flow-mediated dilatation (online supplementary figures 1–13). All funnel plots were asymmetrical, indicating publication bias.
Sensitivity analysis
Results from the sensitivity analysis are summarised in online supplementary table 8. Heterogeneity was reduced in 16/33 outcomes. The most considerable reductions were in those outcomes with fewer studies such as short-term systolic and diastolic blood pressure and long-term total and high-density lipoprotein cholesterol, and these results could alter the main findings. However, in the outcomes with more studies (eg, total cholesterol, high-density lipoprotein cholesterol), it is unlikely that this sensitivity analysis will alter the main findings.
Supplementary file 35
GRADE analysis
All outcomes were rated as very low or low quality evidence demonstrating that the estimate of effect for those outcomes is uncertain.
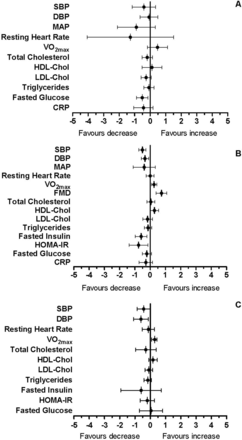
A summary of the change observed for each outcome at all durations is presented as mean difference and 95% CI in figure 3.
Summary graphs. CRP, C reactive protein; DBP, diastolic blood pressure; FMD, flow-mediated dilatation; HDL, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein cholesterol; MAP, mean arterial pressure; SBP, systolic blood pressure. Data are presented as standardised mean differences with 95% confidence intervals. A: Short-term; B: Medium-term; C: Long-term RET.
Resting blood pressure and heart rate
Resting blood pressure and resting heart rate are presented in table 3 and online supplementary figures 14–17. Favourable reductions in systolic blood pressure (in the range 3–5 mm Hg; p≤0.04) and diastolic blood pressure (in the range 1–5 mm Hg; p≤0.008) were apparent after medium-term and long-term term RET interventions (table 1), with studies showing moderate to substantial heterogeneity (I2 range of 64%–86%). There were non-significant effects for mean arterial pressure and resting heart rate after short-term and medium-term RET interventions (table 3 and online supplementary figures 16–17).
Effects of short-term (ST), medium-term (MT) and long-term (LT) RET on resting blood pressure, mean arterial pressure and resting heart rate
V̇O2max
The effect of RET on V̇O2max is presented in online supplementary figure 18. There was an improvement in V̇O2max with RET and moderate heterogeneity (mean difference 2.07 (95% CI 0.75 to 3.39) mL/kg/min, p=0.002; χ²=11.35, I²=30%, p=0.18) in short-term studies (n=9; resistance arm: n=177; control arm: n=131). In medium-term studies (n=48; resistance arm: n=767; control arm: n=687), there was a significant improvement in V̇O2max with RET and substantial heterogeneity (mean difference 1.07 (95% CI 0.38 to 1.76) mL/kg/min, p=0.002; χ²=160.15, I²=71%, p<0.00001). In long-term studies (n=11; resistance arm: n=213; control arm: n=186), there was a significant improvement in V̇O2max with RET (mean difference 1.22 (95% CI 0.44 to 2.0) mL/kg/min, p=0.002; χ²=10.22, I²=2%, p=0.42).
Supplementary file 22
Flow-mediated dilatation
Eight studies reported flow-mediated dilatation; however, due to missing data, only six studies (resistance arm: n=68; control arm: n=70), all medium term, were included in the meta-analysis (online supplementary figure 19). There was a significant improvement in flow-mediated dilatation favouring RET (1.69 (0.97 to 2.41), p<0.0001) with low heterogeneity (χ²=0.72, I²=0%, p=0.98). One short-term study25 and one long-term study26 reported improvements in flow-mediated dilatation after RET.
Supplementary file 23
Blood biomarkers
Blood biomarkers are presented in table 4 and online supplementary figures 20–27. Non-significant effects were observed for total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides and C reactive protein across the different study durations (supplementary figures 20–23 and 27). Significant reductions in fasted insulin (p=0.002) and HOMA-IR (p=0.02) were evident after medium-term but not long-term RET interventions. There was a significant reduction in fasted glucose after medium-term (p=0.02) but not short-term or long-term RET interventions.
Effects of short-term (ST), medium-term (MT) and long-term (LT) RET on blood biomarkers
Subgroup analyses
When comparing healthy young adults ≤40 years (n=44) with healthy older adults ≥41 years (n=50), there was a greater magnitude of cardiometabolic benefit from RET in the older populations (supplementary tables 5 and 6). There were significant reductions in systolic blood pressure with medium-term RET interventions for healthy older adults compared with healthy younger adults (−4.36 (−5.73 to –2.99) mm Hg, p<0.00001 vs −0.56 (−1.57, 0.44) mm Hg, p=0.27, respectively) (supplementary tables 5 and 6). In the healthy older adults, there were significant improvements in systolic blood pressure, diastolic blood pressure, mean arterial pressure, resting heart rate, total cholesterol, high-density lipoprotein cholesterol, triglycerides, fasted insulin, fasted glucose and C reactive protein following medium-term interventions compared with younger adults for the same intervention duration. Significant improvements after long-term interventions were also apparent for diastolic blood pressure, V̇O2max, total cholesterol and fasted glucose in healthy older adults ≥41 years compared with younger adults.
There were greatest improvements in medium-term low-density lipoprotein cholesterol, short-term and medium-term V̇O2max, and short-term systolic and diastolic blood pressure among older adults (≥41 years) with elevated cardiometabolic risk or cardiometabolic disease (n=42) after medium-term interventions, compared with healthy older adults. For example, the largest reduction in systolic blood pressure following medium-term RET interventions was observed in older adults ≥41 years with elevated cardiometabolic risk or disease (−8.80 (−9.90 to –7.69) mm Hg, p<0.00001) compared with the healthy older adults (−4.36 (−5.73 to –2.99) mm Hg, p<0.00001).
Adverse events
One hundred and twenty-three RCTs (71%) did not report the occurrence of adverse events. Fifty studies (29%) reported information on adverse events and 17 of these reported that no adverse events occurred. Of the 50 studies reporting adverse events, 16 studies reported more than one adverse event occurring. Musculoskeletal injuries (eg, lower back pain, knee pain) as a result of the intervention were reported in 20 studies (n=20/50; 40%), with more than one adverse event being reported in 15 of the 20 studies. Two studies (4%) detailed discomfort and muscle soreness related to RET. Illness or injury unrelated to RET were reported in seven (14%) studies. Three studies (6%) reported that participants suffered injuries but the details and whether they were related to the intervention were unclear. Syncope, possibly related to the intervention, was reported in three studies (6%). Cardiac issues (eg, myocardial infarction, angina) thought to be unrelated to the RET were reported in four (8%) studies. Respiratory problems, unrelated to the intervention, were reported in two (4%) and hypoglycaemia in a further two (4%) studies. Four studies (8%) identified participants who underwent elective surgery unrelated to the study. Five studies (10%) reported a newly diagnosed condition or change in medication. Other adverse events reported only once included death (car crash), cerebral stroke, abdominal hernia and deep vein thrombosis; these were not associated with RET. Personal or professional issues resulting in withdrawal from the programme were reported in five (10%) studies.
Discussion
Resistance exercise training had a positive impact on cardiometabolic health, via improvements in resting blood pressure, V̇O2max and blood biomarkers of cardiometabolic risk. These improvements were most convincing for medium-term (7–23 weeks) interventions, which is likely to reflect the higher volume of published studies compared with short-term (<6 weeks) and long-term (≥24 weeks) intervention durations. Relatively few studies have primarily investigated the cardiometabolic health benefits of RET in clinical populations, particularly those at elevated risk of cardiovascular events. There is limited evidence of adverse events associated with RET with only 12% of studies included in the review reporting musculoskeletal injuries. Other studies reported transient levels of muscle soreness following RET, which is common after unaccustomed muscular exercise.27–29 Therefore, we suggest that RET is a safe exercise option for both healthy and clinical populations.
There was a positive effect of RET on systolic and diastolic blood pressure. The reductions observed are of similar magnitude to those after aerobic exercise interventions30–33 and could suggest a dose–response relationship for interventions of varying durations. Furthermore, given that hypertension is a global cause of mortality,34 the pronounced effects of RET on blood pressure outcomes in older populations observed in our subgroup analyses suggest that RET could be an effective non-pharmacological strategy for the prevention and/or control of hypertension in older adults who are at elevated cardiometabolic risk.
The effect of RET on mean arterial pressure and resting heart rate was not statistically significant. Although resting heart rate may be less sensitive to change after RET, the lack of effect on mean arterial pressure (particularly for medium-term studies) could be due to few studies reporting mean arterial pressure in comparison with systolic or diastolic blood pressure. Additionally, diastolic blood pressure has a greater influence on mean arterial pressure than systolic blood pressure and, due to the less pronounced effect of RET on diastolic blood pressure, this could have impacted on the significance of mean arterial pressure.
Low cardiopulmonary fitness has an indirect effect on cardiovascular disease risk and is partially (40%–60%) mediated by cardiovascular risk factors including hypertension, hypercholesterolaemia, obesity and fasting glucose.35 Therefore, the beneficial effects of RET on V̇O2max is important. Traditionally, RET has not been used to provide a stimulus for improving cardiopulmonary exercise capacity; however, our findings suggest that RET may be a reasonable choice for improving this health outcome. Improvements in V̇O2max after RET were modest (short term: 2.38 (0.76 to 4.00) mL/kg/min; medium term: 1.13 (0.50 to 1.76) mL/kg/min; long term: 1.23 (0.6 to 1.87) mL/kg/min). However, larger effects were observed for older adults at elevated cardiometabolic risk. This is clinically important since it suggests that RET may contribute to reducing the risk of cardiovascular morbidity and mortality in high-risk populations.36 On the other hand, it is also possible that those who participated in RET also increased their participation in aerobic activity. Exercise training outside of RET interventions was generally not monitored and may account for some of the change in V̇O2max after RET.
Endothelial dysfunction is associated with cardiovascular disease and the ageing process. Endothelial dysfunction is linked to a decrease in nitric oxide availability, which can be improved through exercise.37 A deterioration in flow-mediated dilatation of approximately 1% is associated with a 13% increased risk of future cardiovascular events.38 39 We found improvements in endothelial function (flow-mediated dilatation) with RET programmes that lasted 7–23 weeks. This is likely to result from shear stress-induced adaptations in nitric oxide metabolism resulting from muscular contractions, resting heart rate and blood pressure changes during RET.26 Shear-stress-induced adaptations may not be restricted to blood vessels within the active skeletal muscles, as exercise programmes that are performed predominantly with the legs induce improvements in brachial artery flow-mediated dilatation.40 Therefore, RET may be an effective stimulus for improving flow-mediated dilatation, potentially reducing the risk of cardiometabolic disease.
The most favourable changes in blood biomarkers were apparent in short-term and medium-term studies in the pooled analysis. The lower number of longer-term studies may have reduced the level of statistical power required to detect significant changes. We found greater reductions in low-density lipoprotein cholesterol, triglycerides and fasted glucose among older adults. There were also significant reductions in C reactive protein after short-term and medium-term RET among older adults at elevated cardiometabolic risk (table 4 and supplementary table 7). Reductions in C reactive protein, fasted glucose and insulin, and HOMA-IR could have been mediated by the effect of RET on body composition, including an increase in skeletal muscle mass and reduction in fat mass, and the resulting impact on adipokine secretion,15 41 insulin sensitivity42 and glucose transport.43 44 These improvements in metabolic functioning following RET could have important clinical implications for the prevention and treatment of metabolic syndrome, type 2 diabetes mellitus and cardiovascular disease.32 41 45 46
Supplementary file 34
Future studies on RET interventions should monitor or control for the potential confounding influence of aerobic exercise outside of the intervention. It is unclear whether improvements in V̇O2max after RET are more attributable to the cardiopulmonary stimulus of RET leading to improved oxygen transport (via increased cardiac stroke volume) or metabolic adaptations resulting in improved use of oxygen at the level of skeletal muscle. Improvements in V̇O2max following medium-term to long-term programmes of aerobic exercise training tend to be greater and mainly reflect an increase in cardiac stroke volume in previously untrained individuals.47 48 The relative importance of and potential to maximise central, systemic and peripheral adaptations, by altering the characteristics of RET (eg, sets, repetitions, rest, etc), warrants further research. Furthermore, additional high-quality research is also required to formulate the optimal design of a RET programme to promote cardiovascular health and risk factor management in middle-aged and clinical populations.
Limitations
The main findings of this systematic review need to be considered in the context of some key limitations, including restricting the search to two electronic databases, language bias and unexplained statistical heterogeneity for some of the analyses. Publication bias was also evident and is probably attributable to inadequate data analysis, poor methodological quality and/or varying sample sizes of included studies. It is unlikely that selective outcome reporting influenced the funnel plots as 90.6% of the studies were rated as low risk for this outcome. Additionally, poor methodological quality of some of the included studies could have affected the estimates of the outcomes. Although all the included studies were RCTs, few studies adequately reported the randomisation process (n=36), allocation concealment (n=14) or blinding of outcome assessment (n=27). Therefore, many studies were rated as unclear bias in multiple categories, and this may have contributed to the lack of reduction in heterogeneity in the sensitivity analyses. Additionally, some data were not pooled due to lack of access to the mean (SD) scores.
Reporting must improve, as many studies had incomplete descriptions of RET programmes and progression, small sample sizes, inadequate documentation of adherence and lacked detail regarding the timing of blood sampling in relation to the last bout of exercise (potentially influencing circulating levels of blood biomarkers). Improved reporting of trials may also improve the quality of evidence, as all outcomes in this review were graded as either very low or low quality, and higher-quality reporting of outcomes may alter the effect estimates. Authors should follow guidelines when reporting trials such as the TIDieR checklist and guide.49
Studies of varying duration are needed, as the majority included in our systematic review involved medium-term interventions. In addition, data analyses were often based only on participants who successfully completed the training intervention, rather than applying an intention-to-treat analysis. This could have altered the study results.23 50 Finally, the cardiorespiratory fitness level of participants prior to a RET intervention is likely to influence training-induced adaptations, and this should be considered in future research.
Conclusion
RET is a safe and effective exercise modality for inducing improvements in resting blood pressure, flow-mediated dilatation, blood biomarkers of cardiometabolic risk and cardiopulmonary fitness in adults. The effects are more pronounced in older adults (≥41 years) and those with elevated cardiometabolic risk or disease.
What are the new findings?
The results suggest that resistance exercise training (RET) improves several cardiometabolic risk factors; however, the quality of the evidence is low and there are no data on hard clinical end-points.
Improvements in cardiometabolic risk factors are more pronounced in individuals with elevated cardiometabolic risk or disease when compared with younger healthy adults.
Few adverse events have been reported, suggesting that RET is safe.
Supplementary file 3
Supplementary file 5
Supplementary file 6
Supplementary file 7
Supplementary file 8
Supplementary file 9
Supplementary file 10
Supplementary file 11
Supplementary file 12
Supplementary file 13
Supplementary file 14
Supplementary file 15
Supplementary file 16
Supplementary file 17
Supplementary file 18
Supplementary file 19
Supplementary file 20
Supplementary file 21
Supplementary file 24
Supplementary file 25
Supplementary file 26
Supplementary file 27
Supplementary file 28
Supplementary file 29
Supplementary file 30
Supplementary file 31
Supplementary file 32
Supplementary file 33
References
Footnotes
Contributors All authors have made substantial contributions to various elements of the study.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.