Article Text
Abstract
Poor reporting of medical and healthcare systematic reviews is a problem from which the sports and exercise medicine, musculoskeletal rehabilitation, and sports science fields are not immune. Transparent, accurate and comprehensive systematic review reporting helps researchers replicate methods, readers understand what was done and why, and clinicians and policy-makers implement results in practice. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and its accompanying Explanation and Elaboration document provide general reporting examples for systematic reviews of healthcare interventions. However, implementation guidance for sport and exercise medicine, musculoskeletal rehabilitation, and sports science does not exist. The Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science (PERSiST) guidance attempts to address this problem. Nineteen content experts collaborated with three methods experts to identify examples of exemplary reporting in systematic reviews in sport and exercise medicine (including physical activity), musculoskeletal rehabilitation (including physiotherapy), and sports science, for each of the PRISMA 2020 Statement items. PERSiST aims to help: (1) systematic reviewers improve the transparency and reporting of systematic reviews and (2) journal editors and peer reviewers make informed decisions about systematic review reporting quality.
- meta-analysis
- evaluation
- implementation
- methodology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
High-quality systematic reviews deliver quality evidence to readers in a timely fashion—supporting informed decision making in practice.1 Poor reporting of medical and healthcare systematic reviews is prevalent, including in sport and exercise medicine, musculoskeletal rehabilitation and sports science.2 3 Systematic reviews must be clearly, transparently, accurately and comprehensively reported. There must be sufficient detail to allow researchers to replicate methods, for readers to understand what the systematic reviewers have done and why, and for clinicians/practitioners and policy-makers to act.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement, published in March 2021, in five leading biomedical journals4–8—a substantially revised and updated version9 of the PRISMA 2009 Statement10—outlines the minimum items that should be reported in a systematic review to improve transparency and interpretation. The PRISMA Statement is recommended by the EQUATOR Network as the reporting guideline for systematic reviews.
The PRISMA 2020 Statement4 and its accompanying Explanation and Elaboration11 document explain and elaborate general reporting examples for systematic reviews of healthcare interventions, but do not include implementation guidance specific for the context of sport and exercise medicine, musculoskeletal rehabilitation and sports science. Context-specific examples and elaboration are likely to enhance implementation.12 The PRISMA 2020 Statement4 focuses on systematic reviews of interventions, but it can be used as a basis for reporting systematic reviews of other types of research (eg, aetiology, prevalence, prognosis).10
Scope of the Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science guidance
Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science (PERSiST) aims to support systematic reviewers in the sport and exercise medicine (including physical activity), musculoskeletal rehabilitation (including physiotherapy and physical therapy), and sports science fields to implement the PRISMA 2020 Statement4 in their systematic reviews. PERSiST is an implementation document that elaborates12 the PRISMA 2020 items in the context of systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation, and sports science fields, and aims to help: (1) systematic reviewers improve the transparency and reporting of systematic reviews and (2) journal editors and peer reviewers in the relevant fields make informed decisions about systematic review reporting quality.
Methods
In this section, we outline the process for developing the PERSiST guidance and explain how the paper is intended to be used. An expert panel collaborated to produce PERSiST.
Establishing the PERSiST project contributing authors
Nineteen PERSiST team members (working group; 7 women, 12 men) were recruited from the primary research areas of sports medicine (n=13), exercise medicine (n=6), physiotherapy and physical therapy (n=10), musculoskeletal rehabilitation (n=13), physical activity (n=8) and sports science (n=2) (N.B. numbers sum to >19 because working group members each had relevant experience in more than 1 of the primary research areas). We balanced early career researchers (within 7 years of PhD award) (n=9), clinician researchers (n=7) and senior researchers (n=10). All had systematic review methods knowledge and experience, and had contributed to at least one systematic review in their primary research field. Three methodologists, who were members of the group that developed the PRISMA 2009 and 2020 Statements,4 10 served as advisors and provided feedback on the specific examples we proposed. The working group identified appropriate examples; the advisory panel provided input into the structure and organisation of the PERSiST project.
Identifying and appraising examples
We established five teams (three working group members per team, balancing gender, research experience and field of expertise) and allocated up to six of the PRISMA 2020 Statement4 items to each team. Each team was tasked with collaborating to identify exemplary reporting from systematic reviews in sport and exercise medicine (including physical activity), musculoskeletal rehabilitation (including physiotherapy and physical therapy) or sports science. No systematic search was conducted to identify published examples.
Each team sent the examples for each item they were assigned to the project leader who collated them into a single document and circulated it to the working group members for review and feedback. Over two feedback rounds (of 2 weeks each), the 19 working group members considered the examples and provided written feedback via group email. After each feedback round, the PERSiST project leader collated, summarised and synthesised the feedback, and circulated the draft examples. We used a final consensus meeting round (via group email) to decide on the draft examples for all 27 main items and 12 abstract items. The examples were then reviewed by the advisory panel, approved and finalised. Including an example does not imply anything about the overall quality of the full systematic review from where the example was drawn.
How to use the PERSiST guidance
PERSiST complements the primary PRISMA 2020 Statement,4 to help systematic reviewers in sport and exercise medicine, musculoskeletal rehabilitation, and sports science implement the PRISMA 2020 items in their research context. We recommend systematic reviewers use PERSiST alongside the PRISMA 2020 Statement4 and PRISMA 2020 Explanation and Elaboration11 when planning and reporting their systematic review; systematic reviewers may also find the METHODS MATTER statement13 helpful for general guidance on research design, methods and reporting.
PERSiST presents at least 1 exemplar illustrating exemplary reporting for each of the 27 PRISMA 2020 Statement4 items. Some examples were lightly edited for flow, including removing citations or web addresses. In some examples, we highlight additional considerations, share helpful resources and make suggestions for reporting systematic reviews in sport and exercise medicine, musculoskeletal rehabilitation, and sports science—these are boxes headed: Note for systematic reviewers.
Where relevant, we retained the exemplar’s citations or hyperlinks. Citations were renumbered to appear in the PERSiST guidance reference list. The examples are intended to guide systematic reviewers in sport and exercise medicine, musculoskeletal rehabilitation, and sports science regarding what to report—systematic reviewers will make decisions about how to present information (eg, whether to use tables, what information is appropriate for appendices/supplementary files) based on the review content and journal requirements.
Terminology
We adopted and followed the terminology of the PRISMA 2020 Explanation and Elaboration11 (box 1). Most systematic reviews use group level (aggregate) data (eg, mean and SD) from included studies. More sophisticated approaches to data analysis and synthesis include network meta-analysis, individual participant data (IPD) meta-analysis (box 1), umbrella reviews (sometimes called overviews or review of reviews) and prospective meta-analysis.14
Key terms
Systematic review
A systematic review employs specific, systematic methods of searching, selecting, assessing, collating and synthesising evidence to address a clearly formulated review question.44
Systematic review methods aim to minimise bias and maximise the practical relevance of the results to a broader spectrum of end users (eg clinicians, practitioners, patients, athletes, care givers, researchers and policy-makers).
Meta-analysis
Meta-analysis is a statistical technique for synthesising results when appropriate data (eg, effect estimates and their variances) are available, to yield a quantitative summary of the results.80
When making decisions about meta-analysis, consider the available data, methods and clinical differences (heterogeneity) among included studies.44 54 Data pooling using meta-analysis can provide a more precise estimate of treatment effect, diagnostic accuracy or prognosis because of greater statistical power when the results of multiple studies are combined.
Network meta-analysis
Traditional systematic reviews compare two different treatments (or compare one treatment to a control). Network meta-analysis is a statistical technique for synthesising and comparing results from more than two different interventions, even when the interventions have not been directly compared in a trial (the analysis builds a network of interventions based on interventions that have been directly and indirectly compared in different trials).81
Network meta-analysis is a more sophisticated analysis approach because it compares all available interventions, can produce a more precise effect estimate and can rank interventions from most to least effective.81 We recommend collaborating with a statistician to plan and conduct a network meta-analysis.
Individual participant data meta-analysis
Systematic reviewers typically pool aggregate data (eg, group means) extracted from included studies. Individual participant data (IPD) meta-analysis involves systematic reviewers collecting the original data for each participant included in the eligible studies, validating/checking and reanalysing the data.82
Compared with an aggregate data review, synthesising IPD can substantially improve the quantity and quality of data, and ultimately produce a more robust synthesis of the field.82 We recommend collaborating with a statistician to plan and conduct an IPD meta-analysis.
Systematic reviewers who are considering these more sophisticated approaches should visit the PRISMA website (www.prisma-statement.org) for guidance on reporting (including PRISMA Statement extensions for network meta-analysis,15 IPD meta-analysis,16 scoping reviews,17 diagnostic test accuracy,18 reporting of harms19 and health equity studies20 21 and protocols.22 23 Other extensions are in development for: newborn and child health research (PRISMA), rapid reviews (PRISMA), ethically sensitive topics (PRISMA-Ethics), animal research (PRISMA Extension of Preclinical In Vivo Animal Experiments) and outcome measurement instruments (PRISMA-COSMIN).
Implementing the PRISMA 2020 Statement: examples for sport and exercise medicine, musculoskeletal rehabilitation, and sports science
Title
Item 1: title
Identify the report as a systematic review.
Example: ‘Comparative effectiveness of treatment options for plantar heel pain: a systematic review with network meta-analysis.’24
Example: ‘Effectiveness of conservative interventions including exercise, manual therapy and medical management in adults with shoulder impingement: a systematic review and meta-analysis of randomised controlled trials (RCTs).’25
Abstract
Item 2
See the PRISMA 2020 for Abstracts checklist (see list and examples in the summary box at the end of this document).
Introduction
Item 3: rationale
Describe the rationale for the review in the context of existing knowledge.
Example: ‘The International Olympic Committee, among others, has called for more diligence to safeguard the physiological development of the paediatric athlete. Performing a cardiac preparticipation evaluation within paediatric populations is controversial due to a lack of international consensus with regard to when, how and who should undertake such examinations. While data from the USA indicate that paediatric black athletes are particularly susceptible to sudden cardiac death (SCD), there is a general lack of understanding as to which factors (eg, physical growth, race and sex) have the potential to increase the likelihood of generating a false-positive diagnosis and unnecessary disqualification from competitive sport. Consequently, the distinction between paediatric athlete’s heart and cardiac pathology associated with SCD is especially important for this population.’26
Introduction
Item 4: objectives
Provide an explicit statement of the objective(s) or question(s) the review addresses.
Note for systematic reviewers. Frame the objectives of systematic reviews of interventions according to the population, intervention, comparison, outcomes (PICO) framework. For a guide on framing other types of systematic reviews (eg, prognosis, diagnostic test accuracy) we recommend Munn et al. 27
Example: ‘The aim of this systematic review and meta-analysis of randomised trials was to provide a comprehensive overview of the effectiveness of all relevant non-surgical interventions for adults with shoulder impingements and outcomes on impairment (pain and active range of motion), activity limitation or participation restriction (shoulder function questionnaires) based on an a priori stated hierarchy.’25
Example: ‘This review intended to evaluate the effectiveness of exercise compared with other conservative interventions in the management of LET (lateral elbow tendinopathy). We also tried to synthesise the evidence regarding exercise type, mode and dosage aiming to inform clinical practice.’28
Methods
Item 5: eligibility criteria
Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses.
Note for systematic reviewers. Structure the eligibility criteria according to the framework used to define the review objective(s) or question(s) (eg, PICO) to help readers understand the scope of the systematic review.
Example:
’Types of studies
Eligible RCTs were identified from systematic reviews investigating the effects of exercise therapy and published in the Cochrane database of Systematic Reviews. RCTs, cluster-randomised trials and randomised crossover studies were included if they compared an exercise therapy intervention with a non-exercising control treatment.
Types of participants
Studies that included participants with or without a medical condition were eligible, except for participants receiving chemotherapy, as all or nearly all these participants are anticipated to experience adverse events. Otherwise, no studies were excluded based on specific characteristics of the participants.
Types of intervention
Exercise therapy was the main intervention and each exercise session had to include active exercise therapy for at least 50% of the total time. Furthermore, the exercise could not be combined with any pharmacological, surgical or electrotherapeutic intervention. Besides strength/resistance, aerobic and neuromuscular exercise (defined as exercise interventions targeting sensorimotor deficiencies and functional stability), the following active exercise interventions were also included: dancing, running, cycling, QiGong and Tai Chi. However, interventions like whole body vibration, facial exercises, yoga, stretching or range of motion exercises or bladder training were excluded. There were no restrictions on the setting in which the exercise therapy was performed, that is, classes, gymnasium, etc.
Types of control intervention
Studies with comparators such as a non-exercise therapy control group, usual care, attention intervention, etc were included, as were nutraceuticals, placebo and education (eg, back school and similar interventions). However, studies where the control group involved any exercise (including stretching), pharmacological, surgical or electrotherapeutic intervention were excluded.
Type of outcome measures
The outcomes of interest were measures of adverse events. As classifying adverse events as treatment-related is largely subjective, and with unknown validity, the current study was not focused on reported adverse effects, but on adverse events as any undesirable event occurring during the study, divided into serious and non-serious adverse events.’29
Methods
Item 6: information sources
Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted.
Note for systematic reviewers. Ensure search decisions are tailored to the systematic review—avoid arbitrary decisions (eg, number of information sources to search).
For more information about grey literature, publication bias, and why it is important to search for grey literature in sport and exercise medicine, musculoskeletal rehabilitation, and sports science, see Winters and Weir30 and chapter 4 of the Cochrane Handbook (searching for and selecting studies).31 Searching grey (unpublished) literature identifies (1) ongoing studies that could be included when a systematic review is updated or that may warrant delaying publishing the review to ensure the study can be included, (2) completed studies that are unpublished and may never get published (eg, injury surveillance conducted by sports federations) and (3) books, monographs, dissertations, policy documents, reports etc that may address the systematic review question and provide relevant data. Reporting sources of grey literature will help readers determine the risk of bias due to missing (unpublished) studies.
Collaborate with a medical/healthcare librarian or information specialist—professionals who have extensive training in literature searching—when planning, developing and executing systematic review searches.32 Quality searching includes choosing appropriate databases and other information sources, designing search strategies for databases and registers, executing searches and saving and collating the results, documenting and reporting the search, and updating the search.31 For more information we recommend chapter 4 of the Cochrane Handbook. 31
Example:
’Electronic searches
We […] searched the following databases from inception to 11 March 2016 without restrictions to language or publication status:
Cochrane Central Register of Controlled Trials (CENTRAL, which includes the Cochrane Back and Neck group (CBN) trials register) (The Cochrane Library, 2016, Issue 2).
MEDLINE (OvidSP, 1946 to March week 1 2016; Appendix 1).
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP, 10 March 2016),
Embase (OvidSP, 1980 to 2016 week 10),
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1981 to 11 March 2016),
PsycINFO (OvidSP, 2002 to March week 2 2016),
Allied and Complementary Medicine Database (AMED) (OvidSP, 1985 to March 2016),
CBN Trials Register (Cochrane Register of Studies (CRS))),
Cochrane Complementary Medicine Field Trials Specialized Register (Cochrane Register of Studies Online (CRSO))),
IndMED,
PubMed,
US National Institutes of Health ClinicalTrials.gov,
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
The searches were previously run in 2013 and 2014. In 2014, the ClinicalTrials.gov, WHO ICTRP and a supplementary search of the CBN Specialised Register in the CRS were added to the search strategy. In 2016, the PubMed search was revised to capture studies not in MEDLINE using the strategy recommended by Duffy 2014. The Information Specialist of the CBN conducted all searches except for the Cochrane Complementary Medicine Field Specialised Register, which we searched through the CRSO.
Searching other resources
We screened the reference lists of included studies and contacted experts in the field (eg, authors of included studies) for information on additional trials, including unpublished or ongoing studies.’33
Example: ‘We conducted a comprehensive database search using PubMed, EMBASE, Cochrane Library, SPORTDiscus and PEDro to search clinical practice guidelines (CPGs) that presented rehabilitation of ACL injuries. To search grey literature and CPG repositories we used the OpenGrey, National Guideline Clearinghouse of the Agency for Healthcare Research and Quality, Guidelines International Network and National Institute for Health and Care Excellence (NICE) databases. […] all searches up to the 30 September of 2018 […].’34
Methods
Item 7: search strategy
Present the full search strategies for all databases, registers and websites, including any filters and limits used.
Note for systematic reviewers. The PRISMA Search Reporting Extension (PRISMA-S) and accompanying checklist35 aids quality reporting for search strategies. PRISMA-S outlines how and what to report so others can reproduce the search. Consider seeking peer review from an information specialist or librarian (eg, using the PRESS 2015 Guideline Evidence-Based Checklist36 when developing a search strategy to improve the quality of the search. 37
Example: ‘Twelve systematic searches covering diagnosis, prevention and treatment for each of the four sections (1: hamstring, 2: adductor, 3: rectus femoris/quadriceps and 4: calf) […]. No restrictions were applied concerning year of publication, however, only publications in English were included. We searched individual text words in title and abstract supplemented with Medical Subject Headings (MeSH) terms. We combined anatomical region of interest (eg, ‘Groin (MeSH)’ OR ‘adductor’ OR ‘groin’) AND type of injury (eg, ‘Athletic Injury (MeSH)’ OR ‘Strains and Sprains (MeSH)’ OR ‘strain*’ OR ‘injur*’ OR ‘re-injur*’ OR ‘reinjur*’) AND outcome for diagnosis and treatment domains (eg, ‘Diagnosis (MeSH)’ OR ‘exam*’ AND ‘Return To Sport’ OR ‘full training,’ respectively) OR intervention for prevention domains (eg, ‘Primary Prevention (MeSHs)’ OR ‘Reduc*’). […] A flow chart of searches and the complete search strategy for all searches and databases is available as supplemental.’38
METHODS
Item 8: selection process
Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process.
Example: ‘The selection of studies was a three-stage process, with the identified citations independently evaluated for inclusion by two reviewers. The first stage was evaluation of titles selected with systematic searches described above. The article was included in this first screen if the title identified athletes and/or lumbar discectomy. We then reviewed the abstracts of all articles identified as meeting the search criteria. Full-text articles meeting criteria were retrieved and read independently by both reviewers and assessed for inclusion in the study. Disagreement was resolved by consensus between the two reviewers and a third reviewer if consensus could not be reached.’39
Methods
Item 9: data collection process
Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process.
Example: ‘Two reviewers independently extracted data using a specifically designed standardised data extracting form (see study protocol), and afterwards the reviewers compared the extracted data for consistency. All inconsistencies between the two forms were resolved by discussion between the two data extractors. Any disagreement between the data extractors after the initial discussion related to inconsistencies between the two individual data extractions was to be solved by involving a third person. General study information, participants and intervention characteristics, compliance, adverse events, withdrawals and outcome measures were extracted. Where data were not available from tables or the results section, the authors of the study in question were contacted by email, with one reminder after 2 weeks, if they did not respond to the first email.’40
Methods
Item 10a: data items
List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (eg, for all measures, time points, analyses), and if not, the methods used to decide which results to collect.
Example:
’Outcomes
The guideline panel identified outcomes of importance to patients; we used outcomes from the trials that most closely corresponded to those chosen by the patients (the systematic reviewers worked with 33 patient partners to identify and prioritise the outcomes of interest for the systematic review) (table 1). Because it was the most bothersome symptom for 86% of the patients who completed our survey, we considered pain as the most important outcome in this systematic review.
Patient-important outcomes identified by the guideline panel aligned to the most corresponding outcome set available in the included trials and studies (reproduced with permission)
We defined serious harms as death, bleeding (uncontrolled or requiring transfusion), cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, cerebrovascular accident, acute renal failure, unplanned intubation, requiring ventilator for >48 hours, deep infection (surgical site or organ/space) sepsis, septic shock, pneumonia, wound dehiscence, pulmonary embolism, deep vein thrombosis or peripheral nerve injury.
[…] We used a priori-defined decision rules for data extraction:
When trialists reported final values and change from baseline values for the same outcome, we extracted final values.
When trialists reported unadjusted and adjusted values for the same outcome, we extracted the unadjusted values.
When trialists reported data based on the intention-to-treat (ITT) sample and another sample (eg, per-protocol, as-treated), we extracted ITT-analysed data.
Data management
When trials used different outcome measures to evaluate the same construct, we chose the most common outcome measure as the index and transformed mean differences and SD of other outcome measures to the index instrument, and pooled the data using mean difference as the summary estimate […] For trials not reporting the index instrument, we followed a prespecified outcome hierarchy when deciding which data would be pooled (box 2).’41
Outcome hierarchy (included in supplemental file of the original publication; reproduced with permission)41
Pain
Overall pain.
Average pain in a preceding period.
Unspecified pain.
Pain with activity in a preceding period.
Night pain in a preceding period.
Worst/highest pain in a preceding period.
Night pain in a preceding period.
Rest pain in a preceding period.
Current pain.
(If multiple periods during which the pain was evaluated were available, the shortest was chosen).
Function outcomes and mixed function-capacity-pain scores
Oxford Shoulder Score.
Constant-Murley Score.
American Shoulder and Elbow Surgeons Standardised Form.
UCLA Shoulder Score.
Simple Shoulder Test.
Shoulder Disability Questionnaire.
Neer Score.
Watson-Sonnabend Score.
Health-related quality of life.
EQ-5D.
15D.
EQ-VAS.
SF-36.
Methods
Item 10b: data items
List and define all other variables for which data were sought (eg, participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information.
Note for systematic reviewers. If you have a long and/or detailed list of variables for which data were sought, consider including a summary of the data items (ie, variables other than outcome(s)) that were extracted as an appendix/supplemental file or as a file uploaded to an online repository (eg, Open Science Framework).
Example of list of other variables for which data were sought:
‘We also extracted the following data: trial characteristics, patient demographic variables, diagnosis, treatment and data about trial methodology. Online supplementary appendix table 1 (box 3) presents a full list of extracted data items.’41
The full list of extracted data items (reproduced with permission)41
Trial characteristics.
Study objectives.
Inclusion and exclusion criteria.
Definition of SAPS (subacromial pain syndrome).
Number of patients allocated to intervention and control groups.
Follow-up time.
Sample size estimations.
Study sponsorships and conflict of interest statements and trial registry identifiers.
Patient demographic-related variables
Sex distribution.
Age.
Duration of symptoms.
Severity of symptoms at baseline.
Shape of acromion.
Employment and physical activity participation.
Diagnosis or treatment-related data
Indications for surgery
Indications for other treatments.
Treatments administered (key details).
Concomitant pathology (eg, subacromial bursitis) and the method of diagnosis of the concomitant pathology, especially imaging.
Trial methodology
Information on sequence generation
Allocation concealment.
Degrees and success of blinding.
Completeness of data (loss to follow-up).
Handling of missing data and possible effects.
Intention-to-treat analysis.
Selective reporting and other sources of bias (dissimilarity of patient groups, cointerventions not evenly distributed among the groups, compliance differences, differences in timing of the outcome assessment(s).
Example of explanation for assumptions made about any missing or unclear information:
‘If authors did not report relevant numeric outcome data in the text, we contacted the authors or, when available, extracted the data from figures and graphs’.41
Methods
Item 11: study risk of bias assessment
Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process.
Note for systematic reviewers. For information on assessing risk of bias in the context of systematic reviews in the sport medicine, musculoskeletal rehabilitation, and sports science fields, we recommend Büttner et al.42 43 Some systematic reviewers use the terms ‘quality assessment’ and ‘risk of bias assessment’ interchangeably. These terms are not synonymous. Quality is poorly defined, but is often used to convey how well the research was conducted and reported. Bias refers to systematic deviations from the truth, which can occur due to flaws in research design, conduct, analysis and/or reporting (eg, outcome reporting biases, spin, per-protocol analyses).42–44
We recommend systematic reviewers complete and report a risk of bias assessment using a clinimetric tool that is appropriate for the review question (eg, causation, prediction, diagnosis). Use a risk of bias assessment tool that facilitates domain-based assessment wherever possible. Do not calculate and present a numerical ‘methodological quality’ score when you mean to assess risk of bias (including quality assessment scales or composite reporting scales). When choosing a risk of bias assessment tool, carefully consider the ideal study design to answer the review question, and the key sources of bias that may influence the results of the systematic review.
The most relevant sources of bias differ depending on the study design. Assessing risk of bias requires a careful approach, tailored to the key threats to the internal validity (ie, bias) of the research question the systematic review aims to address. Different tools are appropriate for different study designs, and may include:
The Cochrane ROB 2.0 for assessing bias in randomised controlled trials.
ROBINS-I for assessing bias in non-randomised intervention studies.
PROBAST for assessing bias in prediction modelling studies.
QUIPS for assessing bias in prognostic studies.
QUADAS-II for assessing bias in diagnostic accuracy studies (see PRISMA-DTA Statement). 18
Example: ‘We used the Risk of Bias 2 tool to assess risk of bias for each trial outcome. We assessed risk of bias on the basis of ‘assignment to intervention’ for all five domains: (1) randomisation process, (2) deviations from intended interventions, (3) missing outcome data, (4) outcome measurement and (5) selection of the reported result. An overall risk of bias judgement was made for each outcome and each time point as either ‘low risk’, ‘some concerns’ or ‘high risk’ of bias.
The assessment was performed independently by two reviewers […]. The reviewers did not perform risk of bias assessment or data extraction for publications in which they were involved as an author. Disagreements were resolved via consensus or by a third reviewer […] if necessary.’45
Methods
Item 12: effect measures
Specify for each outcome the effect measure(s) (eg, risk ratio, mean difference) used in the synthesis or presentation of results.
Example:
‘Synthesis of results
For the analysis on benefits, we calculated the effect sizes in the individual studies as standardised mean differences, allowing pooling and comparison of the various outcomes assessed in the individual trials. We estimated the standardised mean difference as the difference between the mean score of the intervention and control groups divided by the pooled SD of the final score. This estimate of the effect size using standardised mean difference has a slight bias overestimating the effect size, and we applied a correction factor to convert the effect size to Hedges’ g.
In the analysis on harms, we transformed the numbers of adverse events into log odds of events, allowing pooling of data from the individual studies. Results are reported as number of adverse events per 1000 procedures with 95% CIs.’46
Methods
Item 13a: synthesis methods
Describe the processes used to decide which studies were eligible for each synthesis (eg, tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item 5)).
Example: ‘We conducted meta-analyses, guided by considerations of bias. For the primary comparison, we pooled data from comparisons at low risk of bias. For the secondary comparison, we pooled data from comparisons irrespective of bias.
We assessed outcomes at 3 months, 6 months, 1 year, 2 years (for which we pooled data up to 3 years if no 2-year data were available), 5 years (we prioritised time points closest to 5 years) and >10 years following randomisation.’41
Example: ‘Studies were stratified by follow-up time categories: <2 years, 2 to 5 years, 5 to 10 years and >10 years […].
Predefined subgroups were (1) patients treated with ACL reconstruction compared with non-operative treatment and (2) skeletally immature patients compared with skeletally mature patients. We accepted skeletal immaturity as defined in the study […]. If skeletal immaturity was not defined in the study, we applied our definition of age under 16 years at injury for all patients.’47
Methods
Item 13b: synthesis methods
Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics or data conversions.
Example: ‘In studies with no ORs presented, the data were transformed to ORs from standard mean difference of muscle strength between the group of participants who developed osteoarthritis and those who did not. Data from adjusted analyses were extracted if available.’48
Example: ‘When trials used different outcome measures to evaluate the same construct, we chose the most common outcome measure as the index and transformed mean differences and SDs of other outcome measures to the index instrument.’41
Example: ‘When not reported, the SD was estimated from the SE of the mean, 95% CI, p value or other methods suggested in the Cochrane Handbook. Means and SDs were estimated from median and range for two studies. Following Cochrane guidelines, for any study that included two different intervention groups (ie, cycling vs walking) and one control group, the sample size in the control group was evenly divided so a comparison could be made to each intervention. We imputed r=0.5 when the correlation of prescores and postscores was required and we performed sensitivity analyses using r values ranging from 0.1 to 0.9.’49
Methods
Item 13c: synthesis methods
Describe any methods used to tabulate or visually display results of individual studies and syntheses.
Example: ‘Injury risk proportions for individual studies and pooled estimates were summarised in forest plots for the following subgroups: woman, man and combined. A pooled estimate for the relative risk of ACL injury in women compared with men was calculated and summarised in a forest plot. Raw injury incidence rates for individual studies and pooled estimates were summarised in forest plots for the following groups and subgroups: woman, man and combined. Pooled incidence rate ratios for women compared with men were calculated and summarised in forest plots.’50
Methods
Item 13d: synthesis methods
Describe any methods used to synthesise results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used.
Example: ‘Random effect models were used as large heterogeneity was expected due to the different approaches used to compare (ie, controls or contralateral leg) and assess knee extensor muscle strength (ie, isometric or isokinetic) as well as different pain and function scores. A standard [Cochran] Q-test was used to test the heterogeneity between studies, and the I2 statistic measuring the proportion of variance attributable to inconsistency was subsequently calculated. […] I2 each to 100% indicate maximal inconsistency between individual study results. Furthermore, the τ2 value expressing the between study variance was estimated.’51
Example: ‘Owing to expected clinical and methodological heterogeneity between the included studies, we used inverse variance random effects models to estimate relative risks with 95% CIs. […] We dealt with statistical heterogeneity using the I2 statistic and prediction intervals. […] Analyses were conducted in either RevMan V.5.4 or Stata V.15.’52
Example: ‘We performed meta-analyses using a random-effects model as heterogeneity was expected in participant, intervention and outcome characteristics. […] A random effects meta-analysis was applied to estimate the overall relative risk of adverse events in the exercise therapy groups compared with comparator groups. Heterogeneity was examined as between-study variance and calculated as the I2 statistic measuring the proportion of variation in the combined estimates due to study variance. An I2 value of 0% indicates no inconsistency between the results of individual trials, and an I2 value of 100% indicates maximal inconsistency. Meta-analyses were performed in STATA (V.16.1) using the ‘meta’ command.’53
Note for systematic reviewers. There are two common scenarios where systematic reviewers may wish to present a stratified analysis:
An analysis based on predefined groups when it is inappropriate for the specific review question to pool the data (eg, present injury incidence separately for women and men).
Discovering during the course of the review (eg, after extracting data) the need to stratify/subgroup data for a meaningful analysis (eg, short-term, medium-term and long-term outcomes of treatment for low back pain). This should not be confused with the expected and prespecified scenario for investigating statistical heterogeneity, where systematic reviewers conduct subgroup analyses to assess what is contributing to statistical heterogeneity.
Methods
Item 13e: synthesis methods
Describe any methods used to explore possible causes of heterogeneity among study results (eg, subgroup analysis, meta-regression).
Note for systematic reviewers. Subgroup analysis involves splitting studies and their associated participant data into separate (smaller) groups, often to compare different groups (eg, compare data from women and men).44 Subgroup analyses can help the systematic reviewer explore sources of heterogeneity, or answer specific questions about predefined groups of patients, different interventions etc. Predefine appropriate subgroups (eg, based on the systematic review question or on known sources of clinical diversity,54 as much as possible to facilitate unbiased and transparent analysis. Any unplanned subgroup analyses should be clearly labelled as such, and their inclusion justified. Beware the loss of statistical power that accompanies subgroup analyses given fewer participants and events in each comparator/group—interpret the results cautiously.
Example: ‘Stratified analyses were performed for men and women. […] Our initial intention was to conduct subgroup analyses on patients with previous knee injury (ie, ACL injury), overweight, or malalignment. However, sufficient data for these analyses where not found, and thus not included in the present study.’48
Example: ‘We further explored between-study heterogeneity by comparing results from studies grouped according to several study level characteristics using stratified meta-analysis and meta-regression. Study level characteristics assessed were age, sex, MRI sequences employed, participation in weight-bearing sports, radiographic knee osteoarthritis, sample size and overall risk of bias. The prevalence estimates of primary compartment-specific outcomes (ie, tibiofemoral and patellofemoral cartilage defects, bone marrow lesions, osteophytes; medial and lateral meniscal tears) were pooled wherever reported and differences between compartments assessed with a two-proportion z-test.’55
Example: ‘Where sufficient trials were identified, meta-regression was undertaken using STATA (metareg command) to explore the impact of the following trial-level characteristics and whether they were associated with greater fall prevention effects:
Trial design: sample size: <20% missing outcome data; type of comparator intervention.
Participant characteristics: average age ≥75 years; control rate of falls; selected at high risk of falls.
Intervention components: included NICE-recommended components; actively provided treatment to address fall-related risk factors; whether adherence was assessed.’56
Methods
Item 13f: synthesis methods
Describe any sensitivity analyses conducted to assess robustness of the synthesised results.
Note for systematic reviewers. Use sensitivity analyses to check the robustness of decisions made when planning the data synthesis. Sensitivity analyses repeat a previous analysis to check whether a decision was reasonable (eg, the first analysis includes all studies; the sensitivity analysis includes only the studies at low risk of attrition bias). Wherever possible, prespecify sensitivity analyses in the systematic review protocol. Sometimes the need for sensitivity analyses only becomes apparent during the review process—ensure any unplanned sensitivity analyses are clearly labelled in the systematic review report. 57
Sensitivity analyses are often confused with subgroup analyses, but they differ in two important ways: (1) sensitivity analyses do not report an effect estimate from the studies excluded from the analysis, and (2) sensitivity analyses are different ways of estimating the same effect; with subgroup analyses, systematic reviewers are comparing estimates from different subgroups.57
Example: ‘We planned a sensitivity analysis for pain at 1 year to assess the impact of attrition bias due to missing data […]. We also planned to assess small study bias by inspecting the distribution of funnel plots, but there were too few trials.’41
Example: ‘We controlled for smoking status and pre-existing diseases by performing additional sensitivity analyses. Analyses were restricted to never smokers, healthy participants, and healthy never smokers.’58
Example: ‘We examined the effects of methodological quality [bias] on the pooled estimate by removing studies that were at high or unclear risk of bias for the domains of blinding and incomplete outcome data. We had also intended to examine the effects of measurement device on the pooled estimate by removing studies that used pedometers, as previous studies suggest that these might be less accurate in detecting steps in people with COPD.’59
Example: ‘We planned to carry out sensitivity analyses on pain and function by examining the effects of: […]including trials with unclear allocation concealment (at risk of selection bias); including trials with an incomplete description of mild to moderate knee osteoarthritis; […] including trials at risk of detection bias (ie, unclear or no blinding of participant for participant-reported outcomes).’60
Methods
Item 14: reporting bias assessment
Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases).
Example: ‘In 15 of 16 included studies, the effect sizes were not reported; instead, the authors were contacted and asked to provide raw data. Therefore, the risk of publication bias was expected to be minor. Nevertheless, publication bias was assessed visually on a funnel plot (the effect size by the inverse of its standard error) and statistically using Egger’s test, the Begg and Mazumdar rank correlation test and the trim-and-fill method. It should be mentioned, however, that other factors, such as study quality and true heterogeneity, can produce asymmetry in funnel plots.’61
Methods
Item 15: certainty assessment
Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome.
Note for systematic reviewers. There are frameworks (eg, GRADE,62 CINeMA63) that help systematic reviewers provide outcome-based recommendations and judge the certainty (ie, how confident they are in the recommendation) of the body of evidence—supporting transparent critical appraisal and communication. We encourage using the descriptor ‘certainty’ instead of ‘quality’ when describing judgements.
Example: ‘Data were synthesised and the quality of evidence were evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group. […] Two authors assessed the quality of evidence for each outcome relating to diagnostic tests (eg, effectiveness), prevention (eg, risk of injury) and treatment (eg, time to return-to-play) according to the approach from the GRADE working group. Agreement was reached by consensus. The quality of evidence was graded as: (1) high, indicating that further research is unlikely to change the confidence in the estimate of effect, (2) moderate, indicating that further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate, (3) low, indicating that further research is very likely have an important impact on the confidence in the estimate of effect and is likely to change the estimate or (4) very low, indicating high uncertainty about the estimate.
The starting quality of evidence was rated as ‘high’ when data were based on either RCTs for treatment and prevention purposes or rated as ‘low’ when based on observational studies. For diagnostic purposes, the starting quality of evidence was rated as high when based on cohort studies (prospective or cross-sectional). Subsequently, the quality of evidence could be downgraded one or two levels (eg, from high to moderate) for each of the following five domains of the GRADE approach: Study limitations (ie, serious risk of bias such as lack of blinding of outcome assessor or other concerns determined to influence the study result), inconsistency (ie, the heterogeneity of the results across studies if more than one study was included for the specific outcome), indirectness (ie, poor generalisability of the findings to the target population, eg, use groin injuries vs acute adductor injuries for prevention, and/or use of a clinically irrelevant outcome in relation to the question, eg, ‘time to end of treatment’ for ‘time to return-to-play’ outcomes), imprecision of the estimates (ie, wide CIs) and the risk of publication bias. Furthermore, the level of evidence for cohort studies could be upgraded due to a large effect, a dose–response relationship or if no effect was found and all plausible confounding factors identified in the study could be expected to increase the effect. An overview of the risk of bias and grading is provided as supplemental material.’38
Note for systematic reviewers . You may choose to pre-define thresholds or criteria for judging each domain tailored to the review context and outcome(s). Specify pre-defined thresholds in a review protocol. We recommend systematic reviewers cautiously select arbitrary thresholds because they may not apply to comparable systematic reviews. One example: a sample size <800 for each meta-analysis (ie, outcome) may be appropriate for consistent continuous patient-reported outcomes (eg, Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire or session rating of perceived exertion), but may be insufficient when pooling data for disease incidence/prevalence or risk differences of complications (eg, incidence of cauda equina syndrome or rotator cuff re-tear), particularly to detect rare events.
Example: ‘The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the strength of evidence. Studies were downgraded if there were issues with risk of bias, consistency, precision or directness of the outcomes. The reasons for downgrading the evidence are outlined in table 2.’64
Criteria for downgrading the quality of outcomes using the grade approach (reproduced with permission)
Results
Item 16a: study selection
Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram (see figure 1 ).
PRISMA 2020 flow diagram template for systematic reviews (reproduced with permission). PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Note for systematic reviewers. When presenting the results of the search, direct readers to the PRISMA flowchart, so the yield at each step is transparent.
Example: ‘Following deletion of duplicates, the literature search yielded a total of 616 abstracts. A total of 557 abstracts were immediately excluded based on the title and abstract screen; 59 articles were obtained in full text and the selection criteria applied. Fifty-one articles were excluded, as they did not compare outcomes between patients who had had revision and primary ACL reconstruction. Finally, eight studies were included for meta-analysis.’65
Results
Item 16b: study selection
Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded.
Example: ‘[…] the remaining 1378 were evaluated in full text. Of these, 161 were excluded due to exercise included in the control group, 139 for reporting results from the same population in another study, 79 for an additional therapy being delivered together with exercise or less than 50% of the intervention being exercise, 69 for the intervention involving vibration therapy, bladder training or other interventions not meeting the inclusion criteria for interventions. Further, 59 were excluded as they were reported in a language other than English, 45 because the full text was not accessible. Lastly, 8 were excluded because data were not extractable, 5 because the thesis was not available and 40 for other reasons.’29
Results
Item 17: study characteristics
Cite each included study and present its characteristics.
Note for systematic reviewers. Report the key characteristics of the included studies to help readers understand how the included studies address the review question(s). The Template for Intervention Description and Replication (TIDieR)66 framework might help systematic reviewers summarise intervention details in intervention systematic reviews.
Example: ‘The final analysis included a total of 555 youth (283 and 272 in concurrent exercise and aerobic exercise training group, respectively). Three studies included the same population, but analysed different parameters. Nine studies recruited obese youth exclusively; whereas the rest targeted both overweight and obese children. Most studies (n=8) included adolescents (aged 13–18 years), one included only children, and one enrolled both children and adolescents. All studies included boys and girls. Sample sizes across studies ranged from 30 to 150, with a mean of 55 participants.
The primary mode of the aerobic exercise training programmes were based on treadmills and cycle ergometers, elliptical trainers, walking and running programmes, and sports participation. The exercise intensity was monitored using either maximum heart rate, or peak oxygen uptake.
For resistance exercise, studies used body weight exercise, free weights, selectorised machines or circuit training. Interventions duration varied from 10 to 48 weeks, with a mean of 30 weeks’67 (figure 2 (labelled table 2 in the original publication) is an example of how one might present study characteristics).
Example of study characteristics presented in a summary table (reproduced with permission).
Results
Item 18: risk of bias in studies
Present assessments of risk of bias for each included study.
Note for systematic reviewers. Please read in conjunction with item 11 explanation text.
Example: ‘Twenty-two trials (76%) were at high risk of bias. We had some concerns about bias in seven trials (24%). No trials were at low risk of bias. In studies that used both the VISA-A score and return to sport as outcome measures, there was no difference in risk of bias between the outcomes. In 48% of the trials, outcome measurement was a source of bias. All other sources of bias were also commonly judged as high risk: the randomisation procedure (21%), deviations from the intended intervention (28%), missing outcome data (28%) and selection of reported results (24%).’45
Results
Item 19: results of individual studies
For all outcomes, present, for each study: (1) summary statistics for each group (where appropriate) and (2) an effect estimate and its precision (eg, CI/credible interval), ideally using structured tables or plots.
Example included in figure format: ‘One low quality study used x-ray to examine the pubic symphyses of athletes with hip/groin pain and those without pain. A reliable grading system quantified the abnormalities, which were present in all the hip/groin pain subjects (9/20 slight, 9/20 intermediate, 2/20 advanced). In contrast, the athletic control subjects had either no (3/20) or slight (17/20) abnormalities seen on x-ray. Another moderate quality study only reported the radiographic findings in the hip/groin pain subjects and not the control subjects. Synthesising the data on x-ray investigations of the pubic symphysis, there is currently limited evidence that x-ray findings differentiate athletes with hip/groin pain from athletes without pain.
Three moderate quality studies examined pubic bone oedema using MRI in athletes with hip/groin pain and controls. Dichotomous data for the presence or absence of bone oedema were extracted and pooled from these studies. The results indicated that there were high odds that participants with bone oedema on MRI would be in the hip/groin pain group with a large effect size; OR=41.63 (95% CI 1.6 to 1096.60). However, there was high heterogeneity demonstrated by this pooled result (I2=88%), and significant sensitivity to the data from the study of Cunningham et al (figure 3 (labelled figures 8 and 9 in the original publication)). The removal of this study data resulted in an OR=8.1 (95% CI 3.1 to 21.2) for the presence of bone oedema in subjects with hip/groin pain, representing moderate evidence, with a large effect size, that bone oedema in the pubic symphysis differentiates athletes with hip/groin pain from those without this pain.’68
Example of how to present effect estimates in forest plots (reproduced with permission). M-H, Mantel-Haenszel.
Results
Item 20a: results of syntheses
For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies.
Note for systematic reviewers. For more information on outcome-based risk of bias assessment, please refer to Büttner et al.42 43 Read in conjunction with item 11 text. Perform separate risk of bias assessments for each outcome. Lumping all outcomes in a single summary risk of bias assessment (study-level assessment) risks spurious overall judgments because study limitations can distort different outcomes in different ways.
Example:
’Risk of bias assessment
For the comparisons of subacromial decompression surgery versus placebo surgery, the risk of bias was low for all outcomes (figure 4). Due to detection bias (all studies), selection bias, attrition bias and selective reporting, for the comparisons of subacromial decompression surgery vs non-surgical treatment, the risk of bias was high for all outcomes except rotator cuff tears.
Example of how to present domain-based risk of bias assessment results (reproduced with permission). ASAD, arthroscopic subacromial decompression.
Primary comparisons
Two trials were at low risk of bias and sufficiently clinically and methodologically homogeneous to allow pooling for the comparison of subacromial decompression surgery plus postoperative rehabilitation versus placebo surgery plus postoperative rehabilitation.’41
Note for systematic reviewers. Present the study and population characteristics (eg, age, sex, n, etc.) for each synthesis, separately. Below is an example of how to report study and population characteristics of contributing studies for each data synthesis.
Example:
’Mental health symptoms and disorders among current elite athletes
Among those, 11 studies reported prevalence data on distress symptoms among 3335 male and female elite athletes (age ranging from 16 to 29 years) from team sports (eg, cricket, football, handball, ice hockey, rugby) and combined Olympic sports (eg, boxing, gymnastics, judo, rowing, swimming).
[…]
Mental health symptoms and disorders among former elite athletes
Among those, eight studies reported prevalence data on distress symptoms among 1686 former male and female elite athletes (age ranging from 34 to 62 years) from team sports (American football, cricket, football, ice hockey, rugby) and combined Olympic sports.’69
Results
Item 20b: results of syntheses
Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (eg, CI/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect.
Example: ‘Three studies reported on previous history of stress fracture and its association with increased risk of future stress fracture. All three studies had similar findings in that athletes with a previous history of fracture were at increased risk of developing a future stress fracture with ORs ranging from 2.90 to 6.36. An exploratory meta-analysis confirmed the individual study results with runners with a previous history of stress fracture at five times higher risk of a future stress fracture (OR 4.99; 95% CI 2.91 to 8.56; p<0.001; I2=0%. […] Females were at 2.3 times higher risk compared with males (OR 2.31; 95% CI 1.24 to 4.29; p<0.008; I2=0%).’70
Results
Item 20c: results of syntheses
Present results of all investigations of possible causes of heterogeneity among study results.
Example: ‘Stratified analyses for sex showed an increased risk in both men (OR 1.68, 95% CI 1.10, 2.58; I2=55.5%) and women (OR 1.59, 95% CI 0.94, 2.68; I2=54.3%). Differences in risk between men and women did not reach statistical significance (p=0.87).’48
Results
Item 20d: results of syntheses
Present results of all sensitivity analyses conducted to assess the robustness of the synthesised results.
Example: […] ‘The effect remained similar across all strata included in our prespecified…sensitivity analyses (Table 3) with the exception of the type of comparator intervention. […] The pooled estimates of effect remained similar in all prespecified…sensitivity analyses (figure 5 (labelled Table 3 in the original publication)).’56
Example of how to present results of sensitivity analyses (reproduced with permission).
Results
Item 21: reporting biases
Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed.
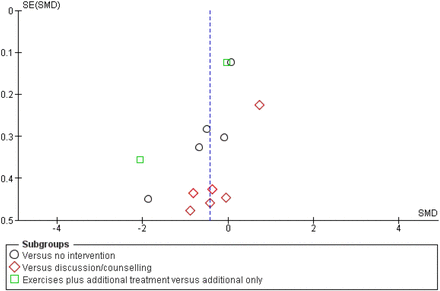
Example: ‘We assessed publication bias for this comparison. The funnel plot (figure 6) shows that small studies with negative effects are missing in the right lower quadrant, which indicates possible publication bias.’71
Funnel plot for assessing publication bias (reproduced with permission). SE, standard error; SMD, standardised mean difference.
Example:
’Publication bias
The funnel plot indicated that almost all studies fell within the expected parameters, most with low SE indicating that most studies were large. A majority of studies reported that women had greater incidence proportion than men. The funnel plot for incidence rate ratio indicated that most studies fell within the expected parameters. Standard error was relatively low, indicating that studies were large, and a majority of studies reported that women were at increased risk of ACL injuries relative to men. The studies are not evenly distributed in the funnel, with studies missing from the lower left quadrant. Studies in the lower left quadrant would represent smaller studies that report a greater incidence proportion or incidence rate of ACL injuries in men compared with women.’50
Results
Item 22: certainty of evidence
Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed.
Note for systematic reviewers. Critical appraisal frameworks (eg, GRADE,62 CINeMA63) facilitate transparent critical appraisal and help systematic reviewers clearly communicate information. We encourage using the descriptor ‘certainty’ instead of ‘quality’ when describing judgements.
Example of GRADE summary reporting and outcome reporting in table format: ‘A summary of the quality of evidence, based on risk of bias, study design, CIs and variability in results, has been collated using the GRADE approach (table 3).
Example of how to present assessments of certainty in the body of evidence for each outcome
All outcomes were rated as very low or low quality [we recommend systematic reviewers use the descriptor ‘certainty’ not ‘quality’] evidence demonstrating that the estimate of effect for those outcomes is uncertain.’64
Discussion
Item 23a: discussion
Provide a general interpretation of the results in the context of other evidence.
Example: ‘There exist several other reviews, although previous reviews have focused on fewer interventions. The most important difference between our systematic review, and previously published reviews is that we have a more stringent assessment of the risk of bias and quality of included trials. This is important because the strength of recommendations (eg, in future guidelines) will be based on the quality of the evidence.
For exercise, our results are in line with the other reviews, with the exception that we concluded that there is only very low-quality evidence where other studies reported moderate or even high or strong evidence. Two reviews evaluated scapula-focused treatments, reporting moderate evidence, and significant but clinically not relevant effects; whereas we did not separately analyse the scapula-focused treatments.’25
Discussion
Item 23b: discussion
Discuss any limitations of the evidence included in the review.
Example: ‘Overall, there was a lack of consistent high-quality evidence to support nominating any particular movement quality outcome as a lower extremity injury risk factor due to inadequate reporting of concepts essential to establishing internal (how well an experiment was carried out) and external (can the results be applied to people and situations beyond the experiment) validity. The biggest threats to internal validity were related to the possibility of selection bias, and the reporting of, and adjustment for, potential influence of factors such as sex, injury history and training exposure.
Specifically, due to the lack of participant characteristic reporting, it was often difficult to determine if the individuals selected for a study differed systematically from those in the source population (selection bias). Equally important was the consistent omission of the characteristics of those lost to follow-up, which made it impossible to determine if participants lost to follow-up were systematically different from those retained in a study. The inability to determine selection bias not only questions the internal validity of several included studies, it impacts the degree to which the findings of these studies can be generalised to the larger population from which the samples were drawn (external validity).’72
Discussion
Item 23c: discussion
Discuss any limitations of the review processes used; comment on the potential impact of each limitation.
Example: ‘We planned threshold analysis as a quantitative means to assess the robustness of network meta-analysis recommendations to potential limitations in the evidence. We were unable to use this approach because of substantial overlap in credible intervals from the network meta-analysis. Due to overlap in the intervals, no recommendations could be made, which is a fundamental prerequisite to performing a valid threshold analysis. To comply with our protocol, we report threshold results in (supplementary Web appendix), but chose to use GRADE to interpret the evidence. We were not able to evaluate small study bias due to too low number of trials. We found three completed trials in trial registers; two are under review, and the publication status of one trial is unknown.’45
Discussion
Item 23d: discussion
Discuss implications of the results for practice, policy, and make recommendations for future research.
Example: ‘On average, patients with subacromial pain syndrome reported reduced pain, and improved physical function and quality of life following both surgical and non-surgical treatment. However, at up to 5 years, irrespective of treatment, patients continued to report pain of an average of 1.5–3 on a scale of 0–10 points on a Visual Analogue Scale. Clinicians working in primary care who are treating patients with subacromial pain syndrome should be aware that some patients experience prolonged symptoms and consider care strategies to support coping.
A placebo control helps answer the research question ‘is there a benefit of subacromial decompression surgery?’ because it minimises the risk of detection and performance biases. Both sources of bias contribute to overestimation of treatment effects by up to 20%. The largely consistent findings of the unblinded studies leave little doubt of the inference that subacromial decompression surgery provides no important benefit to patients. The current evidence provides no support for subacromial decompression surgery as an intervention providing important benefit for patients with subacromial pain syndrome. High-quality evidence indicates that surgery versus placebo surgery confers no important benefit on pain and function—the outcomes most important to patients. Considering the body of evidence, further head-to-head comparisons of subacromial decompression surgery compared with placebo surgery or non-surgical management with the same population are unlikely to change the results. Policymakers, funders and clinicians should consider these results in their funding and clinical decisions regarding the management of patients with shoulder pain.
Our review was designed to assess the benefits and harms of subacromial decompression surgery for managing subacromial pain syndrome. To date, no trial has demonstrated benefit of surgery for any clinical subgroup. In the future, subgroup claims should be supported by data from well-conducted trials at low risk of bias and the use of established criteria for credibility of subgroup effects, ideally enhanced by IPD meta-analysis.
For harms we welcome well-performed observational studies that specifically report harms after subacromial decompression surgery separately from harms following other types of shoulder surgery. Although the finding of no important benefit of surgery is robust, the root cause of subacromial pain and the underlying pathological process remain uncertain, as does the possible best treatment—if such exists—for subacromial pain syndrome. Network meta-analysis might provide information on this question and provide hypotheses to be tested in future methodologically sound trials. Future triallists investigating any treatment for subacromial pain syndrome should adopt a common set of outcomes, and the outcome measures should be standardised.’41
Other information
Item 24a: registration and protocol
Provide registration information for the review, including register name and registration number, or state that the review was not registered.
Note for systematic reviewers. A systematic review protocol is an important way for readers to check whether the systematic review questions and methods have been pre-specified and followed. The protocol guards against bias introduced when too many decisions are made after the data are analysed (eg, selective reporting and outcome switching). Protocols may be (appropriately) modified during the conduct of a systematic review (ensure the registry record is updated when the protocol is modified).73 The PRISMA-P22 checklist guides systematic reviewers on developing a review protocol.
Example: ‘The study was registered at PROSPERO (ID CRD42015024120).’40
Other information
Item 24b: registration and protocol
Indicate where the review protocol can be accessed, or state that a protocol was not prepared.
Example: ‘[…] publicly available comprehensive study protocol including data extraction forms was uploaded at the following website: http://vbn.aau.dk/files/229186677/The_effect_of_the_FIFA_11_prevention_programmes_on_the_overall_injury_rate_in_football_a_systematic_review_and_meta_analysis_version1_1.pdf’40
Example: ‘This systematic review adhered to the […] review protocol, which was published prospectively.74 We prospectively registered this systematic review in the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42016036788).’47
Note for systematic reviewers. The PROSPERO database (www.crd.york.ac.uk/PROSPERO) is an international database of prospectively registered systematic reviews with a health outcome. In addition to the PROSPERO database, systematic reviewers have a range of options to consider when deciding where to register a systematic review. See box 4 for an overview.
Options for prospectively registering systematic review protocols
Preprint servers
Preprint servers are repositories of preliminary reports of scientific work that are yet to complete a full peer review process. The content of preprint servers precedes the formal peer-review, typesetting, copyediting and publishing processes of scientific journals. Currently, there is no cost to post a manuscript on a preprint server.
Preprints are available open access and are typically assigned a digital object identifier (DOI) by the server’s administrator. The DOI protects authors’ intellectual property and allows researchers to disseminate and cite work in manuscripts, grant applications and curricula vitae.
medRχiv (www.medrxiv.org) accepts work in the medical, clinical and related health science fields. SportRχiv (www.sportrxiv.org) accepts work in the sport, exercise, performance and health research fields. OSF Preprints is another alternative for systematic reviewers wishing to obtain a DOI.
Open Science Framework
The Open Science Framework (OSF) is an online community, curated by the Center for Open Science, that supports researchers to collaborate and communicate. The platform allows researchers (individuals and teams) to collate all resources, files, data, statistical code and study protocols for a project. Edits to a project are timestamped, and public projects are searchable. Registering the work creates a time-stamped, read-only version of a project, which can serve as a pre-registered protocol because the files in a registration cannot be modified (the files in a project can be modified).
Registered reports
Registered Reports are an alternative publishing format that elevates the research question and study methods. Approximately 275 biomedical journals offer Registered Reports as a manuscript submission type. Registered Reports facilitate peer review of study protocols, and the work is provisionally accepted based on the research protocol (ie, before the results are known).
The peer review process for Registered Reports occurs in two stages: after the study is designed (protocol) and (for protocols that are ‘accepted in principle’) after the data are collected, analysed and interpreted (full manuscript). The purpose of Registered Reports is to ensure authors’ reporting of their work is consistent with the registered protocol. Registered Reports ensures quality methods (via protocol peer review) and a guarantee that the journal will publish the final version of the systematic review, provided the authors remain true to the protocol.
Institution repositories
Many university libraries offer repositories with the capacity to assign a DOI to authors’ work at the preprint and postprint stages.
Publishing the protocol
Some journals publish systematic review protocols (eg, BMJ Open, Systematic Reviews). Journals typically do not consider protocols for publication once data extraction has commenced. Published protocols usually require prospective registration of the systematic review with an appropriate platform (eg, PROSPERO database or OSF).
Other information
Item 24c: registration and protocol
Describe and explain any amendments to information provided at registration or in the protocol.
Example:
’Change between the protocol and published review
Following data extraction, as the majority of studies reported patient-reported pain separately from function, rather than reporting complete composite scores (eg, KOOS-5), we modified our analysis plan to more comprehensively report pain, function and quality of life outcomes separately. Similarly, the majority of studies reported either 6-month or 12-month outcome data (not both). Therefore, to provide a more complete picture of early, medium and long-term outcomes, we modified the time point at which outcomes were report to: under 6 months, 6–12 months and over 12 months, respectively. One included study did not perform MRI in all patients prior to randomisation .75
Example:
’Deviations from study registration and study protocol
‘Agreement by raters on risk of bias decisions for the included randomised controlled studies was calculated as a percentage of agreement and κ values, and included in the results. Since the secondary analysis concerning type of programme showed that only the FIFA 11+ prevention programme was effective in reducing injuries, all secondary outcomes concerning lower limb, hamstring, knee and ankle injuries were only analysed in relation to this programme.
Furthermore, a post hoc analysis on hip/groin injury in relation to this programme was also included. Preplanned secondary analyses on the incidence rate ratio in the following subgroups: gender (male and female), and mean age groups (youth (<19 years), seniors (19–30 years), old girls/boys (31–39 years) and veterans (>39 years)) were not conducted, as the included studies did not allow making meaningful comparisons with only six studies, where studies with male (n=3) and female participants (n=3) significantly differed in the age group they targeted. The predefined secondary analysis of compliance at team level was not performed as all team-level data could not be obtained from the corresponding authors of the included studies. Instead, the preplanned analysis of the association between prevention programme compliance and injury incidence was further supported by a post hoc analysis of the association between prevention programme compliance and the overall injury incidence rate ratio from each study to accommodate for the risk of substantial variance in injury incidence between studies due to other factors than the FIFA injury prevention programmes.’40
Other information
Item 25: support
Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review.
Example: ‘This research received no grant from any funding agency in the public, commercial or not-for-profit sectors. […] is supported and funded by the National Osteoporosis Society via the Linda Edwards Memorial PhD Studentship.’76
Other information
Item 26: competing interests
Declare any competing interests of review authors.
Example: ‘All authors have completed the ICMJE uniform disclosure form at (available on request from the corresponding author) and declare: […] has received personal fees from Össur, Flexion Therapeutics, Medivir, Teijin, MerckSerono, Allergan, and Galapagos and is editor-in-chief of Osteoarthritis and Cartilage; […] has received personal fees for lectures and royalties for books from Össur, Finnish Orthopedic Society, Studentlitteratur, and Munksgaard and is an associate editor of Osteoarthritis and Cartilage; no other relationships or activities that may appear to have influenced the submitted work.’46
Other information
Item 27: availability of data, code and other materials
Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review.
Example: ‘…a publicly available comprehensive study protocol including data extraction forms was uploaded at the following website: http://vbn.aau.dk/files/229186677/The_effect_of_the_FIFA_11_prevention_programmes_on_the_overall_injury_rate_in_football_a_systematic_review_and_meta_analysis_version1_1.pdf’ 40
Example: ‘Data availability statement. Data are available in a public, open access repository: https://osf.io/q24xh’ 77 [the authors provide the dataset of included studies (with reasons for excluding studies from meta-analysis), and a list of excluded studies with reasons].
Note for systematic reviewers. We could not find any examples in the sports and exercise medicine literature of authors reporting the statistical code used for analyses. For an example of how to report statistical code, please refer to Supplementary Material 1) of Weideman et al 78
Perspectives
All PRISMA 20204 items apply to systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation, and sports science fields. However, some items require more elaboration to promote implementation. PERSiST is intended to support systematic reviewers to implement PRISMA 2020 in their systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation, and sports science fields, and to promote transparent reporting. Use PERSiST in parallel with the PRISMA 2020 Checklist4 and Explanation and Elaboration,11 and with the METHODS MATTER statement13 (as appropriate). We encourage journal editors and reviewers in the relevant fields to use PERSiST to help them make informed judgements about the quality and transparency of systematic review reporting.
Researchers make many decisions at the various stages of conducting a systematic review—from planning the review, completing the search and analysis, and writing the report.79 Decisions include choosing search terms, which data to extract, data pooling policies, choice of statistical tests, among others.79 Poor reporting makes it impossible for other researchers to replicate a systematic review, and a systematic review that cannot be replicated has little value. PERSiST and the PRISMA 2020 Statement and Checklist4 will help systematic reviewers deliver quality systematic reviews in sport and exercise medicine, musculoskeletal rehabilitation, and sports science.
Summary box. Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 for Abstracts checklist and accompanying examples
Item 1: Identify the report as a systematic review.
Example: ‘Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses.’83
Item 2: Provide an explicit statement of the main objective(s) or question(s) the review addresses.
Example: ‘To systematically review and synthesize the effect of soft braces on pain, and self-reported and performance-based physical function in patients with knee osteoarthritis.’83
Item 3: Specify the characteristics used as criteria for eligibility.
Example: ‘Randomised controlled trials examining the effectiveness of any treatment in patients with both insertional and/or midportion Achilles tendinopathy. We excluded trials with N ≤10 per treatment arm or investigating tendon ruptures.’45
Item 4: Specify the information sources (eg, databases, registers) used to identify studies and the date when each was last searched.
Example: ‘The following electronic databases were searched from inception to April 20, 2016: The Cochrane Central Registry for Controlled Trials (CENTRAL), PubMed, EMBASE, Cumulative Index to Nursing and Allied Health Literature, SPORTDiscus, Web of Science and PEDro.’83
Item 5: Specify the methods used to assess risk of bias in the included studies.
Example: ‘Two reviewers independently extracted data and assessed risk of bias with Risk of Bias Tool V.2. We used Grading of Recommendations, Assessment, Development and Evaluation to appraise the strength of the evidence.’84
Example: ‘We used the Cochrane risk-of-bias tool for randomised controlled trials to assess risk of bias and the Grading of Recommendations, Assessment, Development and Evaluation methodology to grade the certainty of evidence.’28
Item 6: Specify the methods used to present and synthesise results.
Example: ‘We pooled data for meta-analyses by length of follow-up, reported as mean differences or standardised mean differences using random-effects wherever possible, or the fixed-effect model, where appropriate. If a meta-analysis was not possible, we synthesised studies narratively.’85
Example: ‘Where the same outcome was assessed across different intervention types, we reported standardised effect sizes for findings from single-study and multiple-study analyses to allow comparison of intervention effects across intervention types. To ease interpretation of the effect size, we also reported the mean difference of effect sizes for single-study outcomes.’86
Example: ‘Rate ratios with 95% confidence intervals were calculated for rate of falls, risk ratios for dichotomous outcomes and standardised mean difference for continuous outcomes. Data were pooled using a random effects model.’56
Item 7: Give the total number of included studies and participants, and summarise relevant characteristics of studies.
Example: ‘Twenty studies comprising 2375 injuries from 1234 athletes (all males and mean age of 24 years) from different sports were included. Internal (65%) and external loads (70%) were collected in more than half of the studies and the session-rating of perceived exertion and total distance were the most commonly collected metrics. The acute chronic workload ratio was commonly calculated using the coupled method (95%), 1:4 weekly blocks (95%) and subsequent week injury lag (80%). There were 14 different binning methods with almost none of the studies using the same binning categories.’87
Item 8: Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, report the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effect (ie, which group is favoured).
Example: ‘Overall, 42.7% (95% confidence interval (CI): 18%, 69%) of patients passed return to sport (RTS) criteria, and 14.4% (95% CI: 8%, 21%) of those who passed experienced a second ACL injury (graft rupture or contralateral ACL injury). There was a nonsignificant 3% reduced risk of a second ACL injury after passing RTS criteria (risk difference, −3%; 95% CI: −16%, 10%; I2 =74%, p=0.61).’88
Item 9: Provide a brief summary of the limitations of the evidence included in the review (eg, study risk of bias, inconsistency and imprecision).
Example: ‘The evidence rating of the Grading of Recommendations Assessment, Development and Evaluation scale was ‘very low certainty’ due to imprecision and heterogeneity of the pooled risk difference estimate.’88
Item 10: Provide a general interpretation of the results and important implications.
Example: ‘In our living network meta-analysis no trials were at low risk of bias and there was large uncertainty in the comparative estimates. For midportion Achilles tendinopathy, wait-and-see is not recommended as all active treatments seemed superior at 3-month follow-up. There seems to be no clinically relevant difference in effectiveness between different active treatments at either 3-month or 12-month follow-up. As exercise therapy is easy to prescribe, can be of low cost and has few harms, clinicians could consider starting treatment with a calf-muscle exercise programme.’45
Item 11: Specify the primary source of funding for the review.
Example: ‘Funding: This research received a grant from the Dutch Association of Medical Specialists to develop a clinical guideline for the treatment of patients with Achilles tendinopathy. The Dutch Patient Federation is involved in this guideline development and assisted in sending out patient surveys.’
Competing interests: […] led a research project in collaboration with Pfizer (project ended 31 December 2018). Pfizer part-funded a junior researcher. The projects were purely methodological, using historical data on pharmacological treatments for pain relief.’45
Example: ‘Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.’89
Item 12: Provide the register name and registration number.
Example: ‘Prospero registration: CRD42018086467.’
Ethics statements
Patient consent for publication
References
Footnotes
Twitter @clare_ardern, @peanutbuttner, @Renato_Physio, @Sinead_Holden, @francoimpell, @EamonnDelahunt, @DrPaulDijkstra, @DrSMathieson, @CathieSherr, @M_Stamatakis, @Bill_Vicenzino, @jwhittak_physio, @KarimKhan_IMHA, @marinuswinters
Correction notice This article has been corrected since it published Online First. Affiliation 6 has been updated.
Contributors CLA, MW, FB and RA proposed the idea for PERSiST, and planned and coordinated the project.The PERSiST Working Group is: RA, CLA, MCA, FB, ED, HPD, SH, FMI, KMK, SM, MSR, GR, CS, ES, BV, AW, JLW, MW and AAW. RA, MCA, SH, FMI and AW led and coordinated the PERSiST Working Group teams in identifying and appraising examples. The PERSiST advisory panel is: MC, DM and MJP. CLA and MW wrote the first draft of the manuscript; all authors contributed to reviewing, editing and revising the manuscript, and approved the final submitted version. CLA is the project lead and guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests RA, MCA, FB, MC, SH, FMI, SM, MSR, GS, BV, JLW, MW and AAW declare they have no competing interests. CLA was a Deputy Editor (Systematic Reviews) for BJSM from 2016 to 2018. ED, HPD and AW are associate editors for BJSM. MCA was a member of the BJSM editorial board from 2008 to 2020. KMK was Editor-in-Chief of BJSM from 2008 to 2020. He holds no position with the BJSM or the BMJ Group at present (September 2021). DM is Chair of the PRISMA group, led the PRISMA 2009 statement and co-led the PRISMA 2020 statement. MJP co-led the PRISMA 2020 statement. ES was editor of BJSM from 2017 to 2020, and editor-in-chief of BMJ Open Sport & Exercise Medicine from 2019 to 2020. He is a senior adviser to BJSM (September 2021).
Provenance and peer review Not commissioned; externally peer reviewed.