Abstract
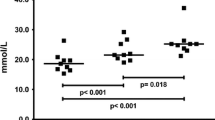
Strenuous exercise has been claimed to modify renal glomerular and tubular function, the relative involvement of the two sites being unknown. These changes may be assessed by the determination of plasma high and low molecular mass proteins. A group of 13 man performed five runs (100, 400, 800, 1,500, 3,000 m) at maximal speed. The excretion rates and renal clearances of creatinine, albumin (Alb), β2-microglobulin (β2-m) and retinol-binding protein (RBP) were determined before and after each run. The glomerular filtration rate remained stable during the shorter runs and declined by about 40% during the longer runs. The excretion rate for Alb rose from 10-fold above the basal value (6 μg·min−1) for the 100 m to 49-fold for the 800 m and then declined for distances up to 3,000 m. The β2-m and RBP had a lesser initial increase, 3.5-(rest 55 ng·min−1) and 7.6-(rest 116 ng·min−1) fold, respectively, for the 100 m run and thereafter showed a higher excretion rate than Alb for the 400 m and 800 m runs. The renal clearances of these high (Alb) and low molecular mass (β2-m and RBP) proteins followed the changes observed for excretion rates. There was a linear relationship (r 2 = 0.996) between plasma lactate concentration and total protein excretion in the postexercise period when taking all five runs into consideration. Glomerular permeability was primarily affected by the 100-m run while the longer runs modified both the glomerular and the tubular sites. To conclude, the present study demonstrated a differential response of the kidney to strenuous exercise with respect to the intensity and duration of the events.
Similar content being viewed by others
References
Abrass CK (1984) Diabetic proteinuria. Am J.Nephrol 4:337–346
Bernard AM, Vyskocil AA, Mahieu P, Lauwerys RR (1987) Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin Chem 33:775–779
Doumas BT, Watson WA, Briggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31:87–96
Everall PH, Wright GH (1958) Low pressure ultrafiltration of protein containing fluids. J Med Lab Technol 15:209–213
Gutmann I, Wahlefeld AW (1974) L(+)-Lactate point determination with lactate dehydrogenase and NAD. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 1464–1468
Hardwicke J, Cameron JS, Harrison JF, Hulme B, Soothill JF (1970) Proteinuria, studied by clearances of individual macromolecules. In: Manuel Y, Revillard, JP, Betuel H (eds) Proteins in normal and pathological urine. Karger, Basel, pp 111–152
Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D (1979) Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int 16:251–270
Mancini G, Carbonara AO, Heremans JF (1965) Immunochemical quantification of antigens by single radial immunodiffusion. Immunochemistry 2:225–254
Mark DD, Zimmer A (1967) Atlas of clinical laboratory procedures, clinical chemistry. McGraw-Hill, New York
Masson PL, Cambiaso CL, Collet-Cassart D, Magnusson CGM, Richards CB, Sindic CJM (1981) Particle counting immunoassay (PACIA). In: Langone JJ (ed) The methods in enzymology. Academic Press, New York, pp 106–139
Peterson PA, Evrin PE, Berggard I (1969) Differentiation of glomerular, tubular and normal proteinuria:determinations of urinary excretion of beta-2-microglobulin, albumin and total protein. J Clin Invest 48:1189–1198
Poortmans JR (1984) Exercise and renal function. Sports Med 1:125–153
Poortmans JR, Van Calck B (1978) Renal glomerular and tubular impairment during strenuous exercise in women. Eur J Clin Invest 8:175–178
Poortmans JR, Labilloy D (1988) The influence of work intensity on postexercise proteinuria. Eur J Appl Physiol 57:260–263
Poortmans JR, Brauman H, Staroukine M, Verniory A, Decaestecker C, Leclercq R (1988) Indirect evidence of glomerular/tubular mixed-type postexercise proteinuria in healthy humans. Am J Physiol 254:F277-F283
Sawicki PT, Berger M (1994) Measuring progression of diabetic nephropathy. Eur J Clin Invest 24:651–655
Sumpio BE, Maack T (1982) Kinetics, competition, and selectivity of tubular absorption of proteins. Am J Physiol 243:F379-F392
Van Beaumont W (1973) Red cell volume with changes in plasma osmolarity during maximal exercise. J Appl Physiol 35:47–50
Yatzidis H (1977) New colorimetric method for quantitative determination of protein in urine. Clin Chem 23:811–812
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Poortmans, J.R., Mathieu, N. & de Plaen, P. Influence of running different distances on renal glomerular and tubular impairment in humans. Eur J Appl Physiol 72, 522–527 (1996). https://doi.org/10.1007/BF00242285
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00242285