Summary
The influence of food on release of drug from a modified release capsule of bromocriptine 5 mg (Parlodel SRO) and a conventional formulation of bromocriptine 5 mg has been studied in 8 healthy male volunteers.
Both formulations produced objective and subjective effects, such as orthostatic reactions, nausea, dizziness, vomiting and nasal congestion. The modified release capsule caused fewer side-effects than the normal capsule. Both formulations had less cardiovascular effect in the fed than in the fasting state.
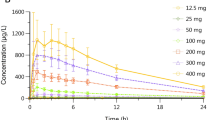
There was no significant difference between the normal and the modified release capsules taken fasting or after a meal in terms of the AUC extrapolated to infinity. The relative bioavailability of the 5 mg modified release capsule was 84.6% of the normal capsule under fasting conditions and 107.5% after food. In contrast to the virtually unchanged extent of absorption, the rate of absorption was markedly affected by food, especially from the conventional capsule. The mean time of 50% absorption increased from 1.06 h (fasting) to 3.2 h (fed), whereas for the modified release capsule food mainly resulted in an increased lag time of absorption.
The almost instantaneous dissolution of bromocriptine from the normal capsule in vitro (both in HCl and fasting human gastric juice) and the delay of absorption after a meal in vivo suggest that the rate limiting step in absorption of the normal capsules is delivery of released drug from the stomach to the small intestine, which is delayed by food.
Both the modified release 5-mg capsule and the normal 5-mg capsule showed extended suppression of prolaction over 36 h, in all subjects, both fasted and after a meal.
Similar content being viewed by others
References
Thorner MO, Flueckiger E, Calne DB (1980) Bromocriptine. A clinical and pharmacological review. Raven Press, New York, pp 55–99
Schran HF, Bhuta SI, Schwarz HJ, Thorner MO (1980) The pharmacokinetics of Bromocriptine in man. In: Goldstein M, Calne DB, Lieberman A, Thorner MO (1980) Ergot compounds and brain function: Neuroendocrine and neuropsychiatrics aspects. Raven Press, New York, pp 125–139
Fimmel CJ, Etienne A, Cilluffo T, v Ritter C, Gasser T, Rey JP, Caradonna-Moscatelli P, Sabbatini F, Pace F, Buehler HW, Bauerfeind P, Blum AL (1985) Long-term ambulatory gastric pH monitoring: Validation of a new method and effect of H2-antagonists. Gastroenterology 88: 1842–1851
Mojaverian P, Ferguson RK, Vlasses PH, Rocci ML, Oren A, Fix JA, Caldwell LJ, Gardner C (1985) Estimation of gastric residence time of the Heidelberg Capsule in humans: Effect of varying food composition. Gastroenterology 88: 392–397
Melander A (1978) Influence of food on the bioavailability of drugs. Clin Pharmacokinet 3: 337–351
Skelly JP (1986) Issues and controversies involving controlled-release drug product studies. Pharmacy Int 1986: 7: 280–286
Woodhouse NJY, Niles N, McDonald D, McCorkell S (1985) Prolactin levels in pregnancy, comparisons of normal subjects with patients having micro or macro adenomas after early bromocriptine withdrawal. Horm Res 21: 1–9
Rosenthaler J, Munzer H, Voges R (1983) Immunoassay of bromocriptine and specificity of antibody: Criteria for choice of antiserum and marker compound. In: Reid E, Leppard JP (eds) Drug metabolite isolation and determination. Plenum Publishing, New York, pp 215–223
Sheiner LB (1981) Program for the extended least squares fit to individual pharmacokinetic data. User's manual, February 1981. Division of Clinical Pharmacology, University of California, San Francisco, USA
Gibaldi M, Perrier D (1982) Pharmacokinetics. Marcel Dekker, New York Basle
RS/1 User's Guide (1983) BBN Research Systems, Bold Beranek and Newman, Cambridge, Mass, USA
Davis SS, Hardy JG, Fara JW (1986) Transit of pharmaceutical dosage forms through the small intestine. Gut 27: 886–892
Meyer JH, Mayer EA, Jehn D, Gu Y, Fink AS, Fried M (1986) Gastric processing and emptying of fat. Gastroenterology 90: 1176–1187
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Drewe, J., Mazer, N., Abisch, E. et al. Differential effect of food on kinetics of bromocriptine in a modified release capsule and a conventional formulation. Eur J Clin Pharmacol 35, 535–541 (1988). https://doi.org/10.1007/BF00558250
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00558250