Abstract
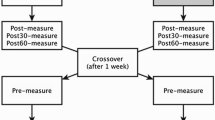
Training a muscle group in one limb yields strength gains bilaterally—the so-called cross-education effect. However, to date there has been little study of the targeted application of this phenomenon in a manner relevant to clinical rehabilitation. For example, it may be applicable post-stroke, where hemiparesis leads to ankle flexor weakness. The purpose of this study was to examine the effects of high-intensity unilateral dorsiflexion resistance training on agonist (tibialis anterior, TA) and antagonist (plantarflexor soleus, SOL) muscular strength and H-reflex excitability in the trained and untrained limbs. Ankle flexor and extensor torque, as well as SOL and TA H-reflexes evoked during low-level contraction, were measured before and after 5 weeks of dorsiflexion training (n = 19). As a result of the intervention, dorsiflexor maximal voluntary isometric contraction force (MVIC) significantly increased (P < 0.05) in both the trained and untrained limbs by 14.7 and 8.4%, respectively. No changes in plantarflexor MVIC force were observed in either limb. Significant changes in H-reflex excitability threshold were also detected: H@thresh significantly increased in the trained TA and SOL; and H@max decreased in both SOL muscles. These findings reveal that muscular crossed effects can be obtained in the ankle dorsiflexor muscles and provide novel information on agonist and antagonist spinal adaptations that accompany unilateral training. It is possible that the ability to strengthen the ankle dorsiflexors bilaterally could be applied in post-stroke rehabilitation, where ankle flexor weakness could be counteracted via dorsiflexor training in the less-affected limb.



Similar content being viewed by others
References
Aagaard P (2003) Training-induced changes in neural functions. Exerc Sport Sci Rev 31:61–67
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Halkjær-Kristensen J, Dyhre-Poulsen P (2000) Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol 89:2249–2257
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92:2309–2318
Abe T, DeHoyos DV, Pollock ML, Garzarella L (2000) Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 81:174–180
Bawa P, Chalmers GR, Stewart H, Eisen AA (2002) Responses of ankle extensor and flexor motoneurons to transcranial magnetic stimulation. J Neurophysiol 88:124–132
Bird SP, Tarpenning KM, Marino F (2005) Designing resistance training programmes to enhance muscular fitness: a review of the acute programme variables. Sports Med 35:841–851
Buchthal F, Schmalbruch H (1970) Contraction times of twitches evoked by H-reflexes. Acta Physiol Scand 80:378–382
Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M (1999) Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol 81:129–139
Carolan B, Cafarelli E (1992) Adaptations in coactivation after isometric resistance training. J Appl Physiol 73:911–917
Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC (2006) Contralateral effects of unilateral training: evidence and possible mechanisms. J Appl Physiol 101:1514–1522
Christie A, Kamen G (2010) Short-term training adaptations in maximal motor unit firing rates and after hyperpolarization duration. Muscle Nerve 41:651–660
Colson S, Pousson M, Martin A, Van Hoecke J (1999) Isokinetic elbow flexion and coactivation following eccentric training. J Electromyogr Kinesiol 9:13–20
Dobkin B (2003) The clinical science of neurologic rehabilitation, 2nd edn. Oxford University Press, New York
Dragert K, Zehr EP (2009) Rhythmic arm cycling modulates Hoffmann reflex excitability differentially in the ankle flexor and extensor muscles. Neurosci Lett 450:235–238
Duncan P, Zorowitz R, Bates B, Choi J, Glasberg J, Graham G, Katz R, Reker D (2005) Management of adult stroke rehabilitation care. Stroke 36:e100–e143
Farthing JP (2009) Cross education of strength depends on limb dominance: implications for theory and application. Exerc Sport Sci Rev 37:179–187
Farthing JP, Chilibeck PD, Binsted G (2005) Cross education of arm muscular strength is unidirectional in right-handed individuals. Med Sci Sport Ex 37:1594–1600
Farthing JP, Krentz JR, Magnus CRA (2009) Strength training the free limb attenuates strength loss during unilateral immobilization. J Appl Physiol 106:830–836
Fimland M, Helgerud J, Solstad GM, Iversen VM, Leivseth G, Hoff J (2009) Neural adaptations underlying cross-education after unilateral strength training. Eur J Appl Physiol 107:723–730
Folland JP, Williams AG (2007) The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37:145–168
Frigon A, Collins D, Zehr EP (2004) Effect of rhythmic arm movement on reflexes in the legs: modulation of soleus H-reflexes and somatosensory conditioning. J Neurophysiol 91:1516–1523
Gabriel D, Kamen G, Frost G (2006) Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med 36:133–149
Geertsen SS, Lundbye-Jensen J, Nielsen JB (2008) Increased central facilitation an antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J Appl Physiol 105:915–922
Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Malkia E, Kraemer W, Newton R, Alen M (1998) Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 84:1341–1349
Holtermann A, Roeleveld K, Engstrom M, Sand T (2007) Enhanced H-reflex with resistance training is related to increased rate of force development. Eur J Appl Physiol 101:301–312
Hortobagyi T, Hill J, Houmard J, Fraser D, Lambert N, Israel R (1996) Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol 80:765–772
Klimstra M, Zehr EP (2008) A sigmoid function is the best fit for the ascending limb of the Hoffmann reflex recruitment curve. Exp Brain Res 186:93–105
Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM, Collins DF (2006a) Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res 170:1–6
Lagerquist O, Zehr EP, Docherty D (2006b) Increased spinal reflex excitability is not associated with neural plasticity underlying the cross-education effect. J Appl Physiol 100:83–90
Lee M, Carroll TJ (2007) Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med 37:1–14
Loadman PM, Zehr EP (2007) Rhythmic arm cycling produces a non-specific signal that suppresses Soleus H-reflex amplitude in stationary legs. Exp Brain Res 179:199–208
Morita H, Crone C, Christenhuis D, Peterson NT, Nielsen J (2001) Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 124:826–837
Moritani T, deVries H (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Morris S, Dodd K, Morris M (2004) Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehab 18:27–39
Munn J, Herbert R, Gandevia C (2004) Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol 96:1861–1866
Munn J, Herbert R, Hancock M, Gandevia C (2005) Training with unilateral resistance exercise increases contralateral strength. J Appl Physiol 99:1880–1884
Quevedo J, Fedirchuk B, Gosgnach S, McCrea DA (2000) Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol 525:549–564
Reeves N, Maganaris C, Narici M (2005) Plasticity of dynamic muscle performance with strength training in elderly humans. Muscle Nerve 31:355–364
Rossignol S, Dubuc R, Gossard J (2006) Dynamic sensorimotor interactions in locomotion. Physiol Rev 86:89–154
Sackley C, Disler PB, Turner-Stokes L, Wade DT, Brittle N, Hoppitt T (2009) Rehabilitation interventions for foot drop in neuromuscular disease. Cochrane Database Syst Rev 8:CD003908
Schubert M, Curt A, Jensen L, Dietz V (1997) Corticospinal input in human gait: modulation of magnetically evoked motor responses. Exp Brain Res 115:234–246
Scripture EW, Smith TL, Brown EM (1894) On the education of muscular control and power. Studies Yale Psychol Lab 2:114–119
Sheskin DJ (2004) Handbook of parametric and non-parametric statistical procedures, 3rd edn. Chapman & Hall/CRC, Boca Raton
Tanaka R (1974) Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res 21:529–540
Taylor N, Dodd K, Damiano D (2005) Progressive resistance exercise in physical therapy: a summary of systematic reviews. Phys Ther 85:1208–1223
Van Cutsem M, Duchateau J, Hainaut K (1998) Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513:295–305
Zehr EP (2002) Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86:455–468
Zehr EP (2006) Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol 101:1783–1794
Zehr EP, Klimstra M, Dragert K, Barzi Y, Bowden M, Javan B, Phadke C (2007a) Enhancement of arm and leg locomotor coupling with augmented cutaneous feedback from the hand. J Neurophysiol 98:1810–1814
Zehr EP, Klimstra M, Johnson EA, Carroll TJ (2007b) Rhythmic leg cycling modulates forearm muscle H-reflex amplitude and corticospinal tract excitability. Neurosci Lett 419:10–14
Zhou S (2000) Chronic adaptations to unilateral exercise: mechanisms of cross education. Ex Sport Sci Rev 29:177–184
Acknowledgments
The main support for this research was provided by a Grant-in-aid of Research (EPZ) and a doctoral fellowship (KD) from the Heart and Stroke Foundation of Canada (BC & Yukon). Additional funding was provided by the Natural Sciences and Engineering Research Council of Canada (EPZ) and the Michael Smith Foundation for Health Research (EPZ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dragert, K., Zehr, E.P. Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp Brain Res 208, 217–227 (2011). https://doi.org/10.1007/s00221-010-2472-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2472-3