Abstract
Objective
Current clinical guidelines provide a unitary approach to manage sport-related concussion (SRC), while heterogeneity in the presentation of symptoms suggests that subtypes of SRC may exist. We systematically reviewed the available evidence on SRC subtypes and associated clinical outcomes.
Data Sources
Ovid Medline, Embase, PsycINFO, and SPORTDiscus
Eligibility Criteria for Selecting Studies
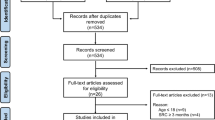
Electronic databases were searched for studies: (i) identifying SRC symptom clusters using classification methodology; or (ii) associating symptom clusters to clinical outcome variables. A total of 6,146 unique studies were identified, of which 75 full texts were independently assessed by two authors for eligibility. A total of 22 articles were included for systematic review.
Data Extraction
Two independent authors performed data extraction and risk of bias analysis using the Cochrane Collaboration tool.
Data Synthesis
Six studies found evidence for existence of SRC symptom clusters. Combining the available literature through Multiple Correspondence Analysis (MCA) provided evidence for the existence of a migraine cluster, a cognitive–emotional cluster, a sleep–emotional cluster, a neurological cluster, and an undefined feelings cluster. Nineteen studies found meaningful associations between SRC symptom clusters and clinical outcomes. Clusters mapping to the migraine cluster were most frequently reported in the literature and were most strongly related to aspects of clinical outcome.
Conclusions
The available literature provides evidence for the existence of at least five subtypes in SRC symptomatology, with clear relevance to clinical outcome. Systematically embedding the differentiation of SRC subtypes into prognosis, clinical management, and intervention strategies may optimize the recovery from SRC.
Similar content being viewed by others
This systematic review and meta-cluster analysis provides robust evidence for the existence of at least five SRC subtypes, identified as a migraine cluster, a cognitive–emotional cluster, a sleep–emotional cluster, a neurological cluster, and an undefined feelings cluster, with clear relevance to clinical outcome. |
The results of this study may pave the way for the transition from a unitary approach to SRC management towards individualized and targeted concussion management and treatment, with the ultimate aim to optimize the recovery of SRC. |
1 Introduction
In the United States alone, an estimated 2.87 million individuals seek care at the Emergency Department for traumatic brain injury each year [1], among which ~ 90% are individuals presenting with mild traumatic brain injury (TBI, i.e. concussion; 2). Concussion as sustained during sports participation accounts for a considerable number of TBI cases, up to 45% in children [3]. A sport-related concussion (SRC) is potent to produce a constellation of acute and post-acute symptoms, incapacitating athletes to return to sport practices [4]. SRC is a heterogeneous injury in terms of etiology and pathophysiology [5, 6] and consequently involves highly variable presentations of symptoms [7]. While current clinical guidelines provide a unitary approach to manage SRC, the heterogeneity in the presentation of SRC symptoms suggests that subtypes of SRC may exist. Subtypes of SRC may be associated with differential clinical outcomes that may require subtype-specific clinical management and treatment.
Diversity in etiology and pathophysiology is thought to be one source of heterogeneity in symptom presentation. SRC may be caused by a direct blow to the head, face, neck, or elsewhere to the body, with linear and rotational acceleration–deceleration forces acting on the brain [8]. Disturbances have been observed at several levels of brain structure and function, among which cellular functioning [9], white matter integrity [10], and functional connectivity [11]. These pathophysiological changes occur diffusely in the brain, while variations in injury characteristics (e.g. force, impact location and direction of skull acceleration–deceleration and rotation) may contribute to marked heterogeneity in SRC phenotypes [4, 5]. The heterogeneity in SRC is also reflected in at least three aspects of clinical outcome: (i) the type of SRC symptoms and functional impairments, (ii) the evolvement of symptoms and impairment over time, and (iii) the recovery trajectory duration.
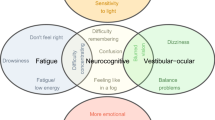
Athletes that sustained a SRC may experience a wide range of subjectively reported symptoms [4], such as physical symptoms (e.g. dizziness, headache), cognitive symptoms (e.g. difficulty concentrating and feeling mentally foggy), sleep/wake-related symptoms (e.g. drowsiness, insomnia), and affective symptoms (e.g. sadness, anxiety). Likewise, a range of objectively measured impairments have been identified after SRC, such as vestibular impairments (e.g. gait unsteadiness), oculomotor impairments (e.g. blurred vision), physical impairments (e.g. amnesia, loss of consciousness), and cognitive impairments (e.g. slowed information processing). The range of potential symptoms and impairments after SRC give rise to a highly individualized nature of SRC-related sequelae [4].
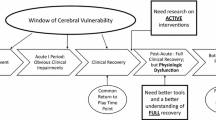
Over time, the presentation of SRC symptoms may also vary during the course of recovery [4, 12]. Certain consequences typically present as on-field impairments immediately after the sustained injury (e.g. loss of consciousness or post-traumatic amnesia), while other symptoms may not become apparent in the first several hours or even days post-injury. Likewise, the duration of recovery from SRC is also subject to distinct variability between athletes [4]. On average athletes recover spontaneously around 10–14 days after concussion [13] with 80–90% experiencing full recovery within one month [13,14,15]. Nevertheless, around 10% of athletes with a SRC remain symptomatic for more than 3 months [16] and are typically diagnosed with persisting symptoms after concussion [17]. It has been shown that the presence of specific symptoms and/or impairments is related to the length of the recovery timeframe and the risk of persisting symptoms after concussion [18,19,20]. Taken together, these findings indicate that in addition to variability in the type and severity of SRC consequences between athletes, there is also notable inter-individual variability in the emergence, evolution, and recovery of symptoms and impairments over time [21].
The evidence on the heterogeneity of etiology and pathophysiology in SRC, resulting in differential symptom presentations and recovery trajectories, indicates that SRC may involve distinct subtypes in which specific symptoms and impairments cluster together. In line with this idea, Collins and colleagues proposed a practice-based model on the delineation of SRC subtypes based on the characteristic symptoms observed at 1 week post-injury, differentiating six subtypes characterized by cognitive/fatigue symptoms, vestibular symptoms, ocular-motor symptoms, anxiety/mood symptoms, post-traumatic migraine symptoms. and cervical symptoms, respectively [22]. Although compelling, the model by Collins and colleagues is practice-based and, therefore, sensitive for bias in the conceptualization of subtypes. Therefore, the model awaits testing in an overview of empirical evidence focusing on data-driven clustering of symptoms into SRC subtypes.
This systematic review aims to evaluate and integrate all available evidence on the classification of SRC symptoms into clusters. Considering that the presentation of particular symptoms is related to prolonged recovery, it is likely that potential subtypes of SRC also relate to differential recovery trajectories. Therefore, we also aim to evaluate the literature on the relation between symptom clusters and clinical outcome. Thereby, this study will reveal the state of the literature with regard to the existence of SRC symptom subtypes and their clinical relevance. The results of this study may pave the way for the transition from a unitary approach to SRC management towards individualized and targeted concussion management and treatment, with the ultimate aim to optimize the recovery of SRC and prevent the development of persisting symptoms after concussion.
2 Methods
This study was performed according to the guidelines set forth by the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group for reporting of systematic reviews of observational studies [23].
2.1 Study Search and Selection
2.1.1 Eligibility Criteria
Studies were considered eligible for this systematic review if they reported on athletes with SRC, and (i) identified SRC symptom clusters using classification methodology, or (ii) examined the association of SRC symptom clusters with clinical outcome variables (e.g. recovery timeframe). Articles were excluded if they: (i) were not peer-reviewed or (ii) represented abstracts of congress presentations.
2.1.2 Information Sources
The search strategy was designed in collaboration with the Amsterdam University Medical Centers (Academic Medical Center location) librarian and involved the following combinations of search terms and their equivalents: Concussion AND Sport AND Symptom assessment (see Online Resource 1 for the specific search queries). The search was performed in the electronic databases Ovid MEDLINE, Embase, PsycINFO, and SPORTDiscus, using both simple search terms and hierarchical family forms (e.g., Medical Subject Headings) and covered all entries between 1946 and 19 December 2018. Furthermore, the reference lists of included articles were hand-searched for additional articles satisfying the inclusion criteria.
2.2 Study Selection
The retrieved records were deduplicated and subsequently screened for eligibility by two reviewers (E.A. and S.L.) based on title and abstract. Relevant records were then independently assessed by the two reviewers based on full texts. Differences in study selection between reviewers were solved by consensus between authors E.A. and S.L.
2.3 Data Extraction
Included studies were systematically reviewed and the following information was extracted from the articles: (i) study design, (ii) study samples, (iii) sample size of patients with SRC and controls, (iv) time of symptom assessment, (v) assessment tools, (vi) methods of analysis, (vii) identified symptom clusters and associations between symptom clusters, and (ix) clinical outcome variables as reported by the authors. Studies identifying SRC symptom clusters using classification methodology were divided in subsections according to their methods of analysis: (1) explorative, data-driven identification of clusters, (2) explorative and confirmative, data-driven identification of clusters and subsequently verification of these clusters, and (3) supportive, collapsing symptoms into clusters as determined by prior research or based on theory/hypothesis and subsequent verification of these clusters in current samples. To provide a systematic aggregation of the available literature, a Multiple Correspondence Analysis (MCA) for clustering of binary data was then conducted on the reported results of SRC symptom clustering using the ‘FactoMineR’ package in R [24, 25]. More specifically, we inserted SRC symptoms as cases in the MCA, while the identified clusters were used as grouping variables. By this procedure, MCA identified clusters of symptoms corresponding to the same grouping variables across the literature. The number of clusters to extract was determined in the eigenvalues histogram. Subsequently, each extracted cluster was labeled according to the set of symptoms that made the strongest contribution to the cluster in terms of effect size (ƞ2). The boundary of this set of symptoms was set at the largest drop in ƞ2 between two subsequent variables in the scree plot (also see Online Resource 2). Clusters from the literature that were found to be associated with clinical outcomes were matched to MCA-identified clusters, based on the highest match in overlapping symptoms between clusters (if the studied cluster contained ≥ 50% of symptoms in one of the MCA clusters).
If available, standardized effect sizes (Cohen’s d, Pearson r correlation) were calculated for the reported effects and interpreted according to Cohen’s guidelines for small (d = 0.2–0.5, r ± 0.1), medium (d = 0.5–0.8, r ± 0.3), and large (d ≥ 0.80, r ≥ 0.5) effect sizes.
2.4 Risk of Bias Analysis
Two independent authors (E.A. and S.L.) assessed the quality of the included studies using the Cochrane Collaboration Tool [26]. As suggested in the handbook, this tool was adapted to enable risk of bias assessment in observational studies. Risk of bias was assessed in terms of selection bias (i.e. representative patient group and adequate case definition), detection bias (i.e. outcome assessor blinding), performance bias (i.e. outcome patient blinding and outcome objectivity), follow-up bias (i.e. follow-up measurements and attrition), and other bias (analysis bias and controls implemented to adjust for confounding).
Each study was scored on all five categories on the level of risk distinguishing between: low risk, high risk, unclear risk, or mixed risk of bias [26]. Overall risk of bias was determined for each study by its total count of high risk and/or unclear risk of bias scores, and half of the total count of mixed risk of bias instances across categories. Relative risk of bias (low relative risk vs. high relative risk) was determined for each study by comparing each study’s overall risk of bias score with the median overall risk of bias score (below vs. above the median, respectively).
3 Results
A Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flow diagram of the study search and selection is displayed in Fig. 1. After deduplication of the 9,593 retrieved records, 6,146 studies remained for further selection. After further assessment of 75 full texts, a total of 22 studies were included for the current systematic review. An overview of study characteristics is provided in Table 1 and a detailed description of risk of bias is displayed in Online Resource 3.
3.1 Symptom Clusters
A total of six studies identified clusters of symptom presentations in athletes with SRC by an explorative analysis (k = 2), an explorative and a confirmative analysis (k = 1), and a supportive analysis (k = 3). All symptom clusters identified by these studies were arbitrarily labeled by the reporting authors based on the symptom loadings on each cluster. An overview of these studies and their main findings is provided in Online Resource 4.
3.1.1 Explorative Evidence
Kontos et al. 2012 investigated symptom clustering in a large independent sample of concussed high school (n = 944) and collegiate (n = 494) athletes within 1 week post-injury [27]. The authors identified four symptom clusters, which consisted of a cognitive–migraine–fatigue cluster, an affective cluster, a somatic cluster, and a sleep–arousal cluster. High school athletes reported lower symptom scores on the sleep–arousal cluster than collegiate athletes, suggesting that an older age may be associated with a higher risk of sleep–arousal symptoms after SRC. Likewise, female athletes reported higher symptom scores on the affective symptom cluster than male athletes, suggesting that female athletes may be at higher risk of affective symptoms after SRC. Moreover, the cognitive–migraine–fatigue cluster showed high cross-loadings on all other clusters, suggesting that this cluster may reflect a primary global cluster emerging within the first week post-injury along with secondary and more specific affective, somatic, and sleep–arousal symptom clusters. This study had low relative risk of bias.
Heyer et al. 2017 investigated symptom clustering in a pediatric population with SRC (n = 510; 28). The authors found seven distinct clusters, including a dizziness–fogginess cluster, emotional cluster, cephalic cluster, drowsiness cluster, somatic cluster, arousal-stimulation cluster, and vomiting cluster emerging both at the day of concussion injury and at the day of clinical evaluation (M = 9.7 ± 7.8 days post-injury). This study had low relative risk of bias.
3.1.2 Explorative and Confirmative Evidence
Joyce et al. 2015 investigated symptom clusters using a large sample of pediatric patients with SRC (n = 420) at an average of 21 days post-injury [29]. The authors identified and confirmed three symptom clusters, consisting of a neurocognitive cluster, somatic cluster, and emotional cluster. This study had low relative risk of bias.
3.1.3 Supportive Evidence
Churchill et al. 2017 investigated a somatic cluster, cognitive cluster, sleep/fatigue cluster and an emotional cluster in 35 university athletes with a concussion and 35 matched controls at an average of 3.6 ± 3.5 days post-injury [30]. The authors found that a somatic cluster and a cognitive cluster were significantly increased at seven days post-injury relative to baseline measurements as well as symptom reports in a control group. This study had high relative risk of bias.
A study by Lau et al. 2011 investigated a migraine cluster, sleep cluster, cognitive cluster, and neuropsychiatric cluster, as determined by a previously published factor analysis [49], in male high school football athletes (n = 108) with SRC within 2–3 days post-injury [31]. The authors confirmed the existence of a migraine cluster, sleep cluster, cognitive cluster, and neuropsychiatric cluster. This study had high relative risk of bias.
A study by Maruta et al. (2018) examined the prevalence of a cognitive–fatigue cluster, a vestibular cluster, an oculomotor cluster, an anxiety/mood cluster, and a migraine cluster, derived through clinical and anecdotal evidence, in 89 athletes at baseline and within 2 weeks of concussion injury [32]. They found that all clusters were more frequently reported by concussed athletes as compared to their baseline measurement. The cognitive–fatigue symptom cluster was most prevalently reported by concussed athletes. This study had high relative risk of bias.
3.1.4 Systematic Aggregation of the Clustering Literature
Taken together, the described findings provide convincing evidence for the existence of clusters of SRC symptoms. To provide a systematic aggregation of the clustering literature, we performed MCA to elucidate the most consistent clustering of symptoms across studies. MCA revealed meta-analytic evidence for the existence of five SRC symptom clusters (Table 2). Based on the most influential set of symptoms in each cluster, we identified a migraine cluster (i.e. headache, sensitivity to light, sensitivity to noise, nausea), a cognitive–emotional cluster (i.e. difficulty remembering, difficulty concentrating, fogginess, feeling more emotional, irritability, feeling slowed down, sadness, nervousness), a sleep–emotional cluster (i.e. trouble falling asleep, sleeping less, feeling more emotional, irritability, sleeping more, sadness, nervousness), a neurological cluster (blurred vision, vomiting, neck pain, pressure in head, visual problems, double vision), and an undefined feelings cluster (“don’t feel right”, confusion).
3.2 Symptom Clusters and Clinical Outcomes
A total of 19 studies investigated associations between SRC symptom clusters and clinical outcome variables. These clusters were matched to the clusters as identified by MCA according to the overlap in symptoms that were captured in each cluster (see Online Resource 5). We discuss the clinical relevance of those clusters that mapped to an MCA cluster, showing a minimum overlap of 50% of symptoms (if the studied cluster contained ≥ 50% of symptoms in one of the MCA clusters). An overview of the studies and their main findings is provided in Table 3. If available, standardized effect sizes were reported.
3.2.1 Migraine Cluster
A total of ten (24%) of reported clusters mapped most closely to the migraine cluster. Clusters mapping to the migraine cluster were found to be associated with prolonged recovery/symptom duration (k = 4), cognitive deficits (k = 3), neuroimaging parameters (k = 2), balance deficits (k = 1), greater total symptom severity scores (k = 1), and provoked vestibular–ocular-motor screening symptoms (k = 1).
3.2.2 Cognitive–Emotional Cluster
A total of eight (19%) of reported clusters had the highest match to the cognitive–emotional cluster. Clusters mapping to the cognitive–emotional cluster were found to be associated with prolonged recovery/symptom duration (k = 4), cognitive and balance deficits (k = 1), neuroimaging parameters (k = 1), greater total symptom severity scores (k = 1), and heart rate recordings (k = 1).
3.2.3 Sleep–Emotional Cluster
Among the reported clusters, nine (21%) mapped to the sleep–emotional cluster. These clusters were found to be associated with prolonged recovery/symptom duration (k = 3), lower sleep quantity (k = 2), cognitive deficits (k = 1), balance deficits (k = 1), greater total symptom severity scores (k = 1), and heart rate recordings (k = 1).
3.2.4 Neurological Cluster
One cluster (2%) with the highest match to the neurological cluster was found to be associated with provoked vestibular–ocular-motor screening symptoms (k = 1).
3.2.5 Undefined Feelings Cluster
None of the reported clusters in the literature had the highest match to the undefined feelings cluster. Therefore, we found no evidence for a relation between this cluster and clinical outcome.
3.2.6 Summary
Clusters reported in the literature mapped most frequently to the migraine cluster (24%), followed by the sleep–emotional cluster (21%), the cognitive–emotional cluster (19%), and the neurological cluster (2%), while none of the remaining clusters in the literature had the highest match to the undefined feelings cluster (0%).
The strength of the relation between symptom clusters and clinical outcomes ranged between small and large, with 28% of the relations showing small effect sizes (relations between the migraine, cognitive–emotional, sleep–emotional cluster and clinical outcomes), 41% of the relations showing moderate effect sizes (relations between the migraine, cognitive–emotional, sleep–emotional cluster and clinical outcomes) and 28% of the relations showing large effect sizes (relations between the migraine cluster and clinical outcomes). These findings suggest that the migraine cluster is most strongly associated with clinical outcome, while no evidence was found for associations between the undefined feelings cluster and clinical outcome. Relations between symptom clusters and clinical outcomes were also consistently observed by studies with low relative risk of bias, indicating that study quality did not account for the observed associations between clusters and clinical outcome measures. Together, these findings suggest that SRC symptom clusters are associated with clinical outcomes, while it remains unclear if and which specific SRC symptom clusters are associated with impairments in particular functional domains.
4 Discussion
This systematic review is the first to (i) systematically evaluate and integrate all available evidence on the classification of SRC symptoms into clusters, (ii) aggregate the available evidence using a meta-analytic approach, and (iii) assess the relation between SRC symptom clusters and clinical outcome. Findings derived from 22 studies representing 5592 athletes with SRC provide strong and consistent evidence for the existence of SRC symptom clusters and relevance of these clusters for clinical outcome. The study findings contribute to our understanding of the distinct heterogeneity of SRC and strongly support a transition from a unitary approach to SRC clinical management towards individualized and targeted SRC prognosis, clinical management, and intervention strategies.
Studies that performed data-driven exploration of symptom clusters provided strong and consistent evidence for clustering of SRC symptoms. The clustering of symptoms was subject to variability between studies, both in the number of clusters (ranging between 2 and 7 cluster solutions) and the symptoms encompassed in the clusters (i.e. the specific symptom clustering together). These variable results may be explained by differences in the methodology of studies (e.g. instrument used for symptom assessment, study sample characteristics, time post-injury). In an attempt to integrate the available evidence and provide an overarching interpretation of symptom clustering across studies, we used MCA to quantitatively investigate the correspondence between the clusters reported in the literature. The results revealed meta-analytic evidence for the most consistently reported clusters, which were identified as a migraine cluster, a cognitive–emotional cluster, a sleep–emotional cluster, a neurological cluster, and an undefined feelings cluster. These findings provide convincing evidence for the existence of (at least) five subtypes in SRC symptomatology.
The observed evidence for the existence of SRC subtypes is in line with the existing literature, such as the practice-based model for delineation of SRC symptoms as proposed by Collins and colleagues [22] that was recently revised in an expert consensus-driven description of concussion subtypes [50]. The current study used data-driven classification to empirically confirm the segregation of SRC symptoms into symptom clusters. With regard to specific subtypes, this study directly confirms the existence of distinct symptom clusters relating to migraine, cognitive, and emotional symptoms [50]. However, in contrast to consensus-driven definitions, we found cognitive symptoms to cluster with emotional symptoms (i.e. cognitive–emotional symptom cluster), sleep symptoms to cluster with emotional symptoms (i.e. sleep–emotional symptom cluster), and ocular symptoms to cluster with vestibular and cervical symptoms (i.e. neurological symptom cluster). The current study extends the consensus-driven description of subtypes by providing a meta-analytic aggregation of all available evidence on the classification of SRC symptoms into clusters, resulting in an empirical taxonomy of symptom clusters, and by assessing the relation between these symptom clusters and clinical outcomes.
Studies included in the current review also support the idea that there are meaningful relationships between SRC symptom clusters and clinical outcomes. More specifically, our findings showed that clusters reported in the literature mapped most frequently to the migraine cluster. Moreover, the effect sizes for the reported associations between the migraine cluster and clinical outcomes were larger compared to the other clusters. Findings also showed that the sleep–emotional cluster, the cognitive–emotional cluster, and the neurological cluster were related to clinical outcome, while no evidence was found for associations between the undefined feelings cluster and clinical outcome. These observed associations between SRC symptom clusters and meaningful clinical outcomes further support the idea that subtypes require targeted clinical management and treatment to improve athlete outcomes.
4.1 Limitations of Available Evidence
The current systematic review has some weaknesses, first determined by the limitations of included studies. Most of these studies had limitations that may have influenced the validity of their findings. For example, only 13.6% of included studies performed explorative data-driven classification of SRC symptom clustering, without prior-formulating these symptom clusters based on hypothesis or other research, and as such, analysis bias may have confounded the identified clusters. Nevertheless this study still aimed to provide a systematic aggregation of SRC clustering, by performing MCA to elucidate the most consistent clustering of symptoms across studies. Moreover, all studies included in this systematic review investigated SRC symptom clustering within the typical phase of recovery (within 1 month post-injury). Consequently, little is known about the classification of SRC symptoms into clusters beyond this recovery phase, which is especially relevant for more complex forms of SRC and the development of persistent symptoms after concussion. Finally, only five of the included studies adjusted for covariates, such as age, sex, and post-injury time, in their analysis, which may also be important confounders and, therefore, may have contributed to bias of the results.
4.2 Implications and Recommendations for Future Research
SRC subtyping may facilitate the symptom-targeted treatment approach through identification of the relevant combination of available treatments (e.g. headache treatment, vestibular treatment, psychological treatment, physical therapy, targeted life style interventions) and the development of novel treatments (e.g. pharmacotherapy, active exercise-based interventions; 4,51,52). It remains unclear, however, how SRC subtypes differentially relate to specific functional impairments. Future studies may importantly contribute by mapping symptom clusters to objective deficits in functional outcome domains measures. This requires the use of a broad battery of functional assessments across domains. Based on our findings, the state of the literature warrants systematic assessment of at least headache characteristics, neurocognitive functioning, emotional functioning, neurological functioning, and sleep. In addition, future studies should further investigate the relation between SRC symptom clustering and variables that might be predictive of the emergence of SRC clusters, such as demographic and injury-related variables [27, 28]. Although it remains unknown to what extent subtypes of concussion are specific to SRC, the current evidence for the clinical relevance of SRC subtyping may also be highly relevant for future research in the broader context of (mild) traumatic brain injury. Since symptoms clusters may overlap and/or co-occur, it could also be valuable to investigate the potential existence of patient subtypes that exhibit a comparable configuration of symptom subtypes. Furthermore, more research is needed with regard to the evolution of SRC (subtypes of) symptoms over time, especially beyond the typical course of recovery (> 1 month; 13,14). An innovative approach to investigate the dynamic inter-relationships among persistent SRC symptoms is a network analysis that was recently proposed by Iverson [53]. This network perspective for persistent symptoms posits that a SRC can be viewed as a set of interacting symptoms in which symptoms co-occur, because they are strongly inter-related, activating, and amplifying. Adopting network analysis in future SRC research may improve our understanding of the temporal dynamics of SRC symptoms.
5 Conclusions
Systematic and meta-analytic evaluation of the literature provides robust evidence for the existence of SRC symptom subtypes. Meta-analysis of the available literature provides evidence for the existence of at least five SRC clusters, identified as a migraine cluster, a cognitive–emotional cluster, a sleep–emotional cluster, a neurological cluster, and an undefined feelings cluster. Clusters mapping to the migraine cluster were most frequently reported in the literature and were most strongly related to aspects of clinical outcome, while there was also evidence for the clinical relevance of the cognitive–emotional, sleep–emotional, and neurological clusters. Taken together, the state of the literature clearly highlights the clinical relevance of SRC symptom subtyping. The results of this study may pave the way for the transition from a unitary approach to SRC management towards individualized and targeted concussion management and treatment, with the ultimate aim to optimize the recovery of SRC and prevent the development of persistent symptoms after concussion.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Peterson A. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths-United States, 2014. Centers for Disease Control and Prevention. U.S. Department of Health and Human Services. 2019. https://www.cdc.gov/TraumaticBrainInjury.
Gerberding JLBS. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to prevent a serious public health problem. Centers Dis Control Prev. 2003.
Sarmiento K, Thomas KE, Daugherty J, Waltzman D, Haarbauer-Krupa JK, Peterson AB, et al. Emergency department visits for sports- and recreation-related traumatic brain injuries among children—United States, 2010–2016. Morb Mortal Wkly Rep. 2019;68(10):237–42.
McCrory P, Meeuwisse W, Dvořák J, Aubry M, Bailes J, Broglio S, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–47.
Kenzie ES, Parks EL, Bigler ED, Lim MM, Chesnutt JC, Wakeland W. Concussion as a multi-scale complex system: an interdisciplinary synthesis of current knowledge. Front Neurol. 2017;8:513.
Bigler ED. Structural neuroimaging in sport-related concussion. Int J Psychophysiol. 2018;132:105–23.
Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TSD, Gioia GA, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports. Neurology. 2013;80(24):2250–7.
Guskiewicz KM, Mihalik JP. Biomechanics of sport concussion: quest for the elusive injury threshold. Exerc Sport Sci Rev. 2011;39(1):4–11.
Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24–33.
Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, et al. A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma. 2012;29(16):2521–38.
McCrea M, Meier T, Huber D, Ptito A, Bigler E, Debert CT, et al. Role of advanced neuroimaging, fluid biomarkers and genetic testing in the assessment of sport-related concussion: A systematic review. Br J Sports Med. 2017;51(12):919–29.
Duhaime A-C, Beckwith JG, Maerlender AC, McAllister TW, Crisco JJ, Duma SM, et al. Spectrum of acute clinical characteristics of diagnosed concussions in college athletes wearing instrumented helmets. J Neurosurg. 2012;117(6):1092–9.
McCrea M, Broglio S, McAllister T, Zhou W, Zhao S, Katz B, et al. Return to play and risk of repeat concussion in collegiate football players: comparative analysis from the NCAA Concussion Study (1999–2001) and CARE Consortium (2014–2017). Br J Sports Med. 2020;54(2):102–9.
Kerr ZY, Zuckerman SL, Wasserman EB, Covassin T, Djoko A, Dompier TP. Concussion symptoms and return to play time in youth, high school, and college American football athletes. JAMA Pediatr. 2016;170(7):647–53.
Zuckerman S, Lee Y, Odom M, Solomon G, Sills A, Forbes J. Recovery from sports-related concussion: Days to return to neurocognitive baseline in adolescents versus young adults. Surg Neurol Int. 2012;3(1):130.
Statista. Percentage of those with a concussion in the U.S. who had post-concussion syndrome from 2010 to 2015. The statistics portal. 2015. https://www.statista.com/statistics/705230/rate-of-post-concussion-syndrome-by-year-in-us/. Accessed 10 Sept 2018.
Ellis MJ, Leddy J, Willer B. Multi-disciplinary management of athletes with post-concussion syndrome: an evolving pathophysiological approach. Front Neurol. 2016;7:136.
Brian L, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216–21.
Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311–8.
Lovell M, Collins M, Iverson G. Recovery from mild concussion in high school athletes. J. Neurosurg. 2003;98(2):296–301.
Reddy CC, Collins MW, Gioia GA. Adolescent sports concussion. Phys Med Rehabil Clin North Am. 2008;19(2):247–69.
Collins MW, Kontos AP, Reynolds E, Murawski CD, Fu FH. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sport Traumatol Arthrosc. 2014;22(2):235–46.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA [Internet]. 2000;283(15):2008–12. https://www.ncbi.nlm.nih.gov/pubmed/10789670. Accessed 27 June 2018.
Team R. RStudio: integrated development for R. Boston: RStudio Inc; 2015.
Husson F, Lê S, Pagès J. Exploratory multivariate analysis by example using R. Exploratory multivariate analysis by example using R. London: Chapman and Hall; 2010.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Kontos AP, Elbin RJ, Schatz P, Covassin T, Henry L, Pardini J, et al. A revised factor structure for the post-concussion symptom scale: Baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375–84.
Heyer GL, Young JA, Fischer AN. Lightheadedness After Concussion: not all dizziness is vertigo. Clin J Sport Med. 2017;11:11.
Joyce AS, Labella CR, Carl RL, Jin-Shei LAI, Zelko FA. The postconcussion symptom scale: utility of a three-factor structure. Med Sci Sport Exerc. 2015;47(6):1119–23.
Churchill NW, Hutchison MG, Graham SJ, Schweizer TA. Symptom correlates of cerebral blood flow following acute concussion. NeuroImage Clin. 2017;16:234–9.
Lau BC, Collins MW, Lovell MR. Sensitivity and specificity of subacute computerized neurocognitive testing and symptom evaluation in predicting outcomes after sports-related concussion. Am J Sports Med [Internet]. 2011 [cited 2016 Jun 19];39(6):1209–16. https://ajs.sagepub.com/lookup/doi/10.1177/0363546510392016. Accessed 10 May 2018.
Maruta J, Lumba-Brown A, Ghajar J. Concussion subtype identification with the rivermead post-concussion symptoms questionnaire. Front Neurol. 2018;9:1034.
Howell DR, O’Brien MJ, Beasley MA, Mannix RC, Meehan WP 3rd. Initial somatic symptoms are associated with prolonged symptom duration following concussion in adolescents. Acta Paediatr. 2016;105(9):e426–e432432.
Howell DR, Kriz P, Mannix RC, Kirchberg T, Master CL, Meehan WP. Concussion symptom profiles among child, adolescent, and young adult athletes. Clin J Sport Med. 2018. https://doi.org/10.1097/JSM.0000000000000629.
Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216–21.
Lau BC, Collins MW, Lovell MR. CutOff scores in neurocognitive testing and symptom clusters that predict protracted recovery from concussions in high school athletes. Neurosurgery. 2011;09:371–9 discussion 379.
Sufrinko AM, Cohen PE, Kontos AP, Marchetti GF, Elbin RJ, Re V. Using acute performance on a comprehensive neurocognitive, vestibular, and ocular motor assessment battery to predict recovery duration after sport-related concussions. Am J Sports Med. 2017;45(5):1187–94.
Guty E, Arnett P. Post-concussion symptom factors and neuropsychological outcomes in collegiate athletes. J Int Neuropsychol Soc. 2018;24:684–92.
Teel EF, Marshall SW, Shankar V, McCrea M, Guskiewicz KM. Predicting recovery patterns after sport-related concussion. J Athl Train. 2017;52(3):288–98.
Brett BL, Kuhn AW, Yengo-Kahn AM, Jeckell AS, Solomon GS, Zuckerman SL. On-field signs predict future acute symptoms after sport-related concussion: a structural equation modeling study. J Int Neuropsychol Soc. 2018;24(5):476–85.
Cohen PE, Sufrinko A, Elbin RJ, Collins MW, Sinnott AM, Kontos AP. Do initial symptom factor scores predict subsequent impairment following concussion? Clin J Sport Med. 2018, pp. 1–8.
Maruta J, Spielman LA, Rajashekar U, Ghajar J. Association of visual tracking metrics with Post-Concussion Symptomatology. Front Neurol. 2018;9:611.
Kontos AP, Elbin RJ, Lau B, Simensky S, Freund B, French J, et al. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. Am J Sports Med. 2013;41(7):1497–504.
Murdaugh DL, Ono KE, Reisner A, Burns TG. Assessment of sleep quantity and sleep disturbances during recovery from sports-related concussion in youth athletes. Arch Phys Med Rehabil. 2018;06:6.
Paniccia M, Verweel L, Thomas SG, Taha T, Keightley M, Wilson KE, et al. Heart rate variability following youth concussion: how do autonomic regulation and concussion symptoms differ over time postinjury? BMJ Open Sport Exerc Med. 2018;4(1):e000355. https://doi.org/10.1136/bmjsem-2018-000355.
Kontos AP, Reches A, Elbin R, Dickman D, Laufer I, Geva AB, et al. Preliminary evidence of reduced brain network activation in patients with post-traumatic migraine following concussion. Brain Imaging Behav. 2016;10(2):594–603.
Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490–6.
Sufrinko A, McAllister-Deitrick J, Collins MW, Kontos AP, Elbin RJ. Family history of migraine associated with posttraumatic migraine symptoms following sport-related concussion. J Head Trauma Rehabil. 2018;33(1):7–14.
Pardini DA, Stump J, Lovell MR, Collins MW, Moritz K, Fu F. The Post concussion symptom scale (PCSS): a factor analysis [abstract]. Br J Sports Med. 2004;38:661–2.
Lumba-Brown A, Teramoto M, Bloom OJ, Brody D, Chesnutt J, Clugston JR, et al. Concussion guidelines step 2: evidence for subtype classification. Neurosurgery. 2019;86(1):2–13.
Collins MW, Kontos AP, Okonkwo DO, Almquist J, Bailes J, Barisa M, et al. Statements of agreement from the targeted evaluation and active management (TEAM) approaches to treating concussion meeting held in Pittsburgh, October 15–16, 2015. Neurosurgery. 2016;79(6):912–29.
Silverberg ND, Iaccarino MA, Panenka WJ, Iverson GL, McCulloch KL, Dams-O’Connor K, et al. Management of concussion and mild traumatic brain injury: a synthesis of practice guidelines. Arch Phys Med Rehabilitat. 2020;101(2):382–93.
Iverson GL. Network analysis and precision rehabilitation for the post-concussion syndrome. Front Neurol. 2019;10:489.
Acknowledgements
The authors would like to thank Joost Daams (Academic Medical Center librarian) for his assistance during the construction of search queries.
Author information
Authors and Affiliations
Contributions
This research represents valid work and every author of this study has made substantial contributions to all aspects of the work. The corresponding author has participated sufficiently in the work to take public responsibility for the entire content of the manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by the Royal Netherlands Football Association (KNVB).
Conflict of Interest
None of the authors had any conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Langdon, S., Königs, M., Adang, E.A.M.C. et al. Subtypes of Sport-Related Concussion: a Systematic Review and Meta-cluster Analysis. Sports Med 50, 1829–1842 (2020). https://doi.org/10.1007/s40279-020-01321-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-020-01321-9