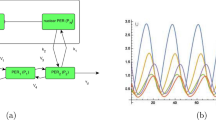
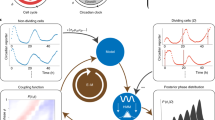
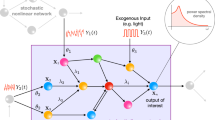
Abstract
Oscillations arise in genetic and metabolic networks as a result of various modes of cellular regulation. In view of the large number of variables involved and of the complexity of feedback processes that generate oscillations, mathematical models and numerical simulations are needed to fully grasp the molecular mechanisms and functions of biological rhythms. Models are also necessary to comprehend the transition from simple to complex oscillatory behaviour and to delineate the conditions under which they arise. Examples ranging from calcium oscillations to pulsatile intercellular communication and circadian rhythms illustrate how computational biology contributes to clarify the molecular and dynamical bases of cellular rhythms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fessard, A. Propriétés Rythmiques de la Matière Vivante (Hermann, Paris, 1936).
Winfree, A. T. The Geometry of Biological Time 2nd edn (Springer, New York, 2001).
Goldbeter, A. Biochemical Oscillations and Cellular Rhythms. The Molecular Bases of Periodic and Chaotic Behaviour (Cambridge Univ. Press, Cambridge, 1996).
Volterra, V. Fluctuations in the abundance of a species considered mathematically. Nature 118, 558–560 (1926).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane currents and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117, 500–544 (1952).
Koch, C. & Segev, I. (eds) Methods in Neuronal Modeling. From Synapses to Networks 2nd edn (MIT Press, Cambridge, MA, 1998).
Keener, J. P. & Sneyd, J. Mathematical Physiology (Springer, New York, 1998).
Noble, D. Modeling the heart—from genes to cells to the whole organ. Science 295, 1678–1682 (2002).
Nicolis, G. & Prigogine, I. Self-Organization in Nonequilibrium Systems. From Dissipative Structures to Order through Fluctuations (Wiley, New York, 1977).
Thomas, R. & d'Ari, R. Biological Feedback (CRC Press, Boca Raton, FL, 1990).
Ferrell, J. E. Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 (2002).
Doedel, E. J. AUTO: A program for the automatic bifurcation analysis of autonomous systems. Cong. Numer. 30, 265–284 (1981). (Available at 〈http://ftp.cs.concordia.ca/pub/doedel/auto/〉.)
Hess, B. & Boiteux, A. Oscillatory phenomena in biochemistry. Annu. Rev. Biochem. 40, 237–258 (1971).
Goldbeter, A. & Caplan, S. R. Oscillatory enzymes. Annu. Rev. Biophys. Bioeng. 5, 449–476 (1976).
Berridge, M. J. Elementary and global aspects of calcium signalling. J. Physiol. (Lond.) 499, 291–306 (1997).
Meyer, T. & Stryer, L. Molecular model for receptor-stimulated calcium spiking. Proc. Natl Acad. Sci. USA 85, 5051–5055 (1988).
Goldbeter, A., Dupont, G. & Berridge, M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc. Natl Acad. Sci. USA 87, 1461–1465 (1990).
De Young, G. W. & Keizer, J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc. Natl Acad. Sci. USA 89, 9895–9899 (1992).
Dupont, G. & Goldbeter, A. Properties of intracellular Ca2+ waves generated by a model based on Ca2+-induced Ca2+ release. Biophys. J. 67, 2191–2204 (1994).
Sneyd, J., Charles, A. C. & Sanderson, M. J. A model for the propagation of intercellular calcium waves. Am. J. Physiol. 266, C293–C302 (1994).
Dupont, G. et al. Mechanism of receptor-oriented intercellular calcium wave propagation in hepatocytes. FASEB J. 14, 279–289 (2000).
Schuster, S., Marhl, M. & Höfer, T. Modelling of simple and complex calcium oscillations. From single-cell responses to intercellular signalling. Eur. J. Biochem. 269, 1333–1355 (2002).
Swillens, S., Dupont, G., Combettes, L. & Champeil, P. From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc. Natl Acad. Sci. USA 96, 13750–13755 (1999).
Spitzer, N. C., Lautermilch, N. J., Smith, R. D. & Gomez, T. M. Coding of neuronal differentiation by calcium transients. BioEssays 22, 811–817 (2000).
De Koninck, P. & Schulman, H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230 (1998).
Gorbunova, Y. V. & Spitzer, N. C. Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature 418, 93–96 (2002).
Dormann, D., Kim, J. Y., Devreotes, P. N. & Weijer, C. J. cAMP receptor affinity controls wave dynamics, geometry and morphogenesis in Dictyostelium. J. Cell Sci. 114, 2513–2523 (2001).
Martiel, J. L. & Goldbeter, A. A model based on receptor desensitization for cyclic AMP signaling in Dictyostelium cells. Biophys. J. 52, 807–828 (1987).
Tang, Y. & Othmer, H. G. Excitation, oscillations and wave propagation in a G-protein-based model of signal transduction in Dictyostelium discoideum. Phil. Trans. R. Soc. Lond. B 349, 179–195 (1995).
Palsson, E. & Cox, E. C. Origin and evolution of circular waves and spirals in Dictyostelium discoideum territories. Proc. Natl Acad. Sci. USA 93, 1151–1155 (1996).
Lauzeral, J., Halloy, J. & Goldbeter, A. Desynchronization of cells on the developmental path triggers the formation of spiral waves of cAMP during Dictyostelium aggregation. Proc. Natl Acad. Sci. USA 94, 9153–9158 (1997).
Bretschneider, T., Siegert, F. & Weijer, C. J. Three-dimensional scroll waves of cAMP could direct cell movement and gene expression in Dictyostelium slugs. Proc. Natl Acad. Sci. USA 92, 4387–4391 (1995).
Chadwick, D. J. & Goode, J. A. (eds) Mechanisms and Biological Significance of Pulsatile Hormone Secretion (Novartis Found. Symp. 227) (Wiley, Chichester, 2000).
Knobil, E. Patterns of hormone signals and hormone action. New Engl. J. Med. 305, 1582–1583 (1981).
Belchetz, P. E., Plant, T. M., Nakai, Y., Keogh, E. J. & Knobil, E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202, 631–633 (1978).
Hindmarsh, P. C., Stanhope, R., Preece, M. A. & Brook, C. G. D. Frequency of administration of growth hormone—an important factor in determining growth response to exogenous growth hormone. Horm. Res. 33 (Suppl. 4), 83–89 (1990).
Tornheim, K. Are metabolic oscillations responsible for normal oscillatory insulin secretion? Diabetes 46, 1375–1380 (1997).
Li, Y. X. & Goldbeter, A. Frequency specificity in intercellular communication: the influence of patterns of periodic signalling on target cell responsiveness. Biophys. J. 55, 125–145 (1989).
Goldbeter, A., Dupont, G. & Halloy, J. The frequency encoding of pulsatility. Novartis Found. Symp. 227, 19–36 (2000).
Wagner, C., Caplan, S. R. & Tannenbaum, G. S. Genesis of the ultradian rhythm of GH secretion: a new model unifying experimental observations in rats. Am. J. Physiol. 275, E1046–E1054 (1998).
Maki, L. W. & Keizer, J. Mathematical analysis of a proposed mechanism for oscillatory insulin secretion in perifused HIT-15 cells. Bull. Math. Biol. 57, 569–591 (1995).
Dunlap, J. C. Molecular bases for circadian clocks. Cell 96, 271–290 (1999).
Young, M. W. & Kay, S. A. Time zones: a comparative genetics of circadian clocks. Nature Rev. Genet. 2, 702–715 (2001).
Reppert, S. M. & Weaver, D. R. Coordination of circadian timing in mammals. Nature 418, 935–941 (2002).
Hardin, P. E., Hall, J. C. & Rosbash, M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540 (1990).
Kronauer, R. E., Forger, D. B. & Jewett, M. E. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the phototopic range. J. Biol. Rhythms 14, 500–515 (1999).
Gonze, D., Roussel, M. & Goldbeter, A. A model for the enhancement of fitness in cyanobacteria based on resonance of a circadian oscillator with the external light-dark cycle. J. Theor. Biol. 214, 577–597 (2002).
Goodwin, B. C. Oscillatory behavior in enzymatic control processes. Adv. Enzyme Regul. 3, 425–438 (1965).
Ruoff, P., Vinsjevik, M., Monnerjahn, C. & Rensing, L. The Goodwin model: simulating the effect of light pulses on the circadian sporulation rhythm of Neurospora crassa. J. Theor. Biol. 209, 29–42 (2001).
Goldbeter, A. A model for circadian oscillations in the Drosophila period protein (PER). Proc. R. Soc. Lond. B 261, 319–324 (1995).
Leloup, J. C. & Goldbeter, A. A model for circadian rhythms in Drosophila incorporating the formation of a complex between the PER and TIM proteins. J. Biol. Rhythms 13, 70–87 (1998).
Tyson, J. J., Hong, C. I., Thron, C. D. & Novak, B. A simple model of circadian rhythms based on dimerization and proteolysis of PER and TIM. Biophys. J. 77, 2411–2417 (1999).
Glossop, N. R., Lyons, L. C. & Hardin, P. E. Interlocked feedback loops within the Drosophila circadian oscillator. Science 286, 766–768 (1999).
Shearman, L. P. et al. Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019 (2000).
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Ueda, H. R., Hagiwara, M. & Kitano, H. Robust oscillations within the interlocked feedback model of Drosophila circadian rhythm. J. Theor. Biol. 210, 401–406 (2001).
Smolen, P., Baxter, D. A. & Byrne, J. H. Modeling circadian oscillations with interlocking positive and negative feedback loops. J. Neurosci. 21, 6644–6656 (2001).
Leloup, J. C. & Goldbeter, A. Towards a detailed computational model for the mammalian circadian clock. Proc. Natl Acad. Sci. USA (submitted).
Richardson, G. S. & Malin, H. V. Circadian rhythm sleep disorders: pathophysiology and treatment. J. Clin. Neurophysiol. 13, 17–31 (1996).
Toh, K. L. et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291, 1040–1043 (2001).
Ebisawa, T. et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2, 342–346 (2001).
Leloup, J. C. & Goldbeter, A. A molecular explanation for the long-term suppression of circadian rhythms by a single light pulse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1206–R1212 (2001).
Barkai, N. & Leibler, S. Circadian clocks limited by noise. Nature 403, 267–268 (2000).
Gonze, D., Halloy, J. & Goldbeter, A. Robustness of circadian rhythms with respect to molecular noise. Proc. Natl Acad. Sci. USA 99, 673–678 (2002).
Vilar, J. M., Kueh, H. Y., Barkai, N. & Leibler, S. Mechanisms of noise-resistance in genetic oscillators. Proc. Natl Acad. Sci. USA 99, 5988–5992 (2002).
Goldbeter, A. et al. From simple to complex oscillatory behavior in metabolic and genetic control networks. Chaos 11, 247–260 (2001).
Rinzel, J. A formal classification of bursting mechanisms in excitable systems. Lect. Notes Biomath. 71, 267–281 (1987).
Olsen, L. F. & Degn, H. Chaos in biological systems. Q. Rev. Biophys. 18, 165–225 (1985).
Glass, L. & Mackey, M.C. From Clocks to Chaos: The Rhythms of Life (Princeton Univ. Press, Princeton, 1988).
Shen, P. & Larter, R. Chaos in intracellular Ca2+ oscillations in a new model for non-excitable cells. Cell Calcium 17, 225–232 (1995).
Kummer, U. et al. Switching from simple to complex oscillations in calcium signaling. Biophys. J. 79, 1188–1195 (2000).
Goldbeter, A. A minimal cascade model for the mitotic oscillator involving cyclin and cdc2 kinase. Proc. Natl Acad. Sci. USA 88, 9107–9111 (1991).
Novak, B. & Tyson, J. J. Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J. Cell Sci. 106, 1153–1168 (1993).
Tyson, J. J. & Novak, B. Regulation of the eukaryotic cell cycle: molecular antagonism, hysteresis, and irreversible transitions. J. Theor. Biol. 210, 249–263.
Tyson, J. J., Chen, K. & Novak, B. Network dynamics and cell physiology. Nature Rev. Mol. Cell Biol. 2, 908–916 (2001).
Elowitz, M. B. & Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000).
Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000).
Lev Bar-Or, R. et al. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc. Natl Acad. Sci. USA 97, 11250–11255 (2000).
Maroto, M. & Pourquié, O. A molecular clock involved in somite segmentation. Curr. Top. Dev. Biol. 51, 221–248 (2001).
Jacquet, M., Renault, G., Lallet, S., de Mey, J. & Goldbeter, A. Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activator Msn2 in Saccharomyces cerevisiae. Nature (submitted).
Boiteux, A., Goldbeter, A. & Hess, B. Control of oscillating glycolysis of yeast by stochastic, periodic, and steady source of substrate: a model and experimental study. Proc. Natl Acad. Sci. USA 72, 3829–3833 (1975).
Termonia, Y. & Ross, J. Oscillations and control features in glycolysis: numerical analysis of a comprehensive model. Proc. Natl Acad. Sci. USA 78, 2952–2956 (1981).
Hynne, F., Dano, S. & Sorensen, P. G. Full-scale model of glycolysis in Saccharomyces cerevisiae. Biophys. Chem. 94, 121–163 (2001).
Reijenga, K. A., Westerhoff, H. V., Kholodenko, B. N. & Snoep, J. L. Control analysis for autonomously oscillating biochemical networks. Biophys. J. 82, 99–108 (2002).
Hartwell, L. H., Hopfield, J. J., Leibler, S. & Murray, A. W. From molecular to modular cell biology. Nature 402 (Suppl.), C47–C52 (1999).
Koshland, D. E. Jr The era of pathway quantification. Science 280, 852–853 (1998).
Petty, H. R. Neutrophil oscillations: temporal and spatiotemporal aspects of cell behavior. Immunol. Res. 23, 85–94 (2001).
Nielsen, K., Sörensen, P. G. & Hynne, F. Chaos in glycolysis. J. Theor. Biol. 186, 303–306 (1997).
Honma, S. et al. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419, 841–844 (2002).
Acknowledgements
I thank G. Dupont, D. Gonze, B. Jacrot, J. C. Leloup and G. Oster for discussions and helpful comments on the manuscript. This work was supported by a grant from the Fonds de la Recherche Scientifique Médicale, Belgium.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goldbeter, A. Computational approaches to cellular rhythms. Nature 420, 238–245 (2002). https://doi.org/10.1038/nature01259
Issue Date:
DOI: https://doi.org/10.1038/nature01259
This article is cited by
-
Systematic analysis of negative and positive feedback loops for robustness and temperature compensation in circadian rhythms
npj Systems Biology and Applications (2023)
-
Radical pairs can explain magnetic field and lithium effects on the circadian clock
Scientific Reports (2022)
-
Stochastic synchronization in nonlinear network systems driven by intrinsic and coupling noise
Biological Cybernetics (2022)
-
Discovering design principles for biological functionalities: Perspectives from systems biology
Journal of Biosciences (2022)
-
Global entrainment in the brain–body–environment: retrospective and prospective views
Biological Cybernetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.