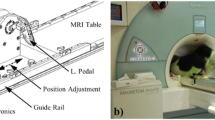
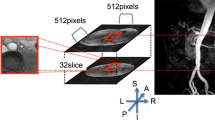
Abstract
Magnetic resonance (MR) imaging techniques and a custom MR-compatible exercise bicycle were used to measure, in vivo, the effects of exercise on hemodynamic conditions in the abdominal aorta of eleven young, healthy subjects. Heart rate increased from 73±6.2 beats/min at rest to 110±8.8 beats/min during exercise (p < 0.0001). The total blood flow through the abdominal aorta increased from 2.9±0.6 L/min at rest to 7.2±1.4 L/min during exercise (p < 0.0005) while blood flow to the digestive and renal circulations decreased from 2.1±0.5 L/min at rest to 1.6±0.7 L/min during exercise (p < 0.01). Infrarenal blood flow increased from 0.9±0.4 L/min at rest to 5.6±1.1 L/min during exercise (p < 0.0005). Wall shear stress increased in the supraceliac aorta from 3.5±0.8 dyn/cm2 at rest to 6.2±0.5 dyn/cm2 during exercise (p < 0.0005) and increased in the infrarenal aorta from 1.3±0.8 dyn/cm2 at rest to 5.2±1.3 dyn/cm2 during exercise (p < 0.0005). © 2002 Biomedical Engineering Society.
PAC2002: 8719Uv, 8761-c
Similar content being viewed by others
REFERENCES
Caro, C. G., J. M. Fitz–Gerald, and R. C. Schroter. Atheroma and arterial wall shear: Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc. R. Soc. London, Ser. B: Biological Sciences 177:109–159, 1971.
Cheng, C. P., D. Parker, and C. A. Taylor. Wall shear stress quantification from magnetic resonance imaging data using lagrangian interpolation functions. In: Proceedings of the 2001 ASME Summer Bioengineering Meeting. Park City, UT, 2001, pp. 795–796.
Cheng, C. P., D. Parker, and C. A. Taylor. Quantification of wall shear stress in large blood vessels using Lagrangian interpolation functions with Cine PC–MRI. Ann. Biomed. Eng. (submitted).
Cybulsky, M. I., and M. A. Gimbrone, Jr.. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251:788–791, 1991.
Fredrickson, J. O., P. Irarrazabal, and N. J. Pelc. Quantitative 3d time–resolved phase contrast MR imaging. SMR Workshop: Cardiovascular MRI: Present and Future, 1994, p. 25.
Fredrickson, J. O., H. Wegmuller, R. J. Herfkens, and N. J. Pelc. Simultaneous temporal resolution of cardiac and respiratory motion in MR imaging. Radiology 195:169–175, 1995.
Friedman, M. H., G. M. Hutchins, and C. B. Bargeron. Correlation between intimal thickness and fluid shear in human arteries. Atherosclerosis (Berlin) 39:425, 1981.
Glagov, S., D. A. Rowley, and R. Kohut. Atherosclerosis of human aorta and its coronary and renal arteries. Arch. Pathol. Lab. Med. 72:558, 1961.
He, X., and D. Ku. Pulsatile flow in the human left coronary artery bifurcation: Average conditions. J. Biomech. Eng. 118:74–82, 1996.
Ku, D., D. Giddens, C. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation: Positive correlation between plaque location and low oscillating shear stress. Atherosclerosis (Berlin) 5:293–302, 1985.
Moore, J., D. Ku, C. Zarins, and S. Glagov. Pulsatile flow visualization in the abdominal aorta under differing physiologic conditions: Implications for increased susceptibility to atherosclerosis. J. Biomech. Eng. 114:391–397, 1992.
Moore, J., and D. Ku. Pulsatile velocity measurements in a model of the human abdominal aorta under resting conditions. J. Biomech. Eng. 116:337–346, 1994.
Moore, J., and D. Ku. Pulsatile velocity measurements in a model of the human abdominal aorta under simulated exercise and postprandial conditions. J. Biomech. Eng. 116:107–111, 1994.
Moore, J., S. Maier, D. Ku, and P. Boesiger. Hemodynamics in the abdominal aorta: a comparison of in vitro and in vivo measurements. J. Appl. Physiol. 76:1520–1527, 1994.
Moore, Jr., J. E., C. Xu, S. Glagov, C. K. Zarins, and D. N. Ku. Fluid wall shear stress measurements in a model of the human abdominal aorta: Oscillatory behavior and relationship to atherosclerosis. Atherosclerosis (Berlin) 110:225–240, 1994.
Niezen, R. A., J. Doornbos, E. E. Van Der Wall, and A. De Roos. Measurement of aortic and pulmonary flow with MRI at rest and during physical exercise. J. Comput. Assist. Tomog. 22:194–201, 1998.
Nobutaka, I., S. Ramasamy, T. Fukai, R. Nerem, and D. G. Harrison. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ. Res. 79:32–37, 1996.
Oshinski, J. N., D. N. Ku, S. Mukundan, Jr., F. Loth, and R. I. Pettigrew. Determination of wall shear stress in the aorta with the use of MR phase velocity mapping. Magn. Reson. Imaging 5:640–647, 1995.
Oyre, S., E. M. Pedersen, S. Ringgaard, P. Boesiger, and W. P. Paaske. In vivo wall shear stress measured by magnetic resonance velocity mapping in the normal human abdominal aorta. Eur. J. Vasc. Endo. Surg. 13:263–271, 1997.
Pedersen, E. M., S. Hsing–Wen, A. C. Burlson, and A. P. Yoganathan. Two–dimensional velocity measurements in a pulsatile flow model of the normal abdominal aorta simulating different hemodynamic conditions. J. Biomech. 26:1237–1247, 1993.
Pedersen, E. M., S. Kozerke, S. Ringgaard, M. B. Scheidegger, and P. Boesiger. Quantitative abdominal aortic flow measurements at controlled levels of ergometer exercise. Magn. Reson. Imaging 17:489–494, 1999.
Pelc, L. R., N. J. Pelc, S. C. Rayhill, L. J. Castro, G. H. Glover, R. J. Herfkens, D. C. Miller, and R. B. Jeffrey. Arterial and venous blood flow: Noninvasive quantitation with MR imaging. Radiology 185:809–812, 1992.
Pelc, N. J., F. G. Sommer, K. C. Li, T. J. Brosnan, R. J. Herfkens, and D. R. Enzmann. Quantitative magnetic resonance flow imaging. Magn. Reson. Q. 10:125–147, 1994.
Pollack, M., and J. Wilmore. Exercise in Health and Disease: Evaluation and Prescription for Prevention and Rehabilitation, 2nd ed. Philadelphia: W. B. Saunders, 1990.
Roberts, J. C., C. Moses, and R. H. Wilkins. Autopsy studies in atherosclerosis: I. Distribution and severity of atherosclerosis in patients dying without any morphologic evidence of atherosclerotic catastrophe. Circulation 20:511–519, 1959.
Schalet, B., C. Taylor, E. Harris, R. Herfkens, and C. Zarins. Quantitative assessment of human aortic blood flow during exercise. Surg. Forum. XLVIII:359–362, 1997.
Sessa, W., K. Pritchard, N. Seydi, J. Wang, and T. Hintze. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ. Res. 74:349–353, 1994.
Sethian, J. A. Level Set Methods and Fast Marching Methods. Cambridge: Cambridge University Press, 1999.
Strong, J. P., G. T. Malcom, C. A. Mcmahan, R. E. Tracy, W. P. Newman Iii, E. E. Herderick, and J. F. Cornhill. Prevalence and extent of atherosclerosis in adolescents and young adults: Implication for prevention from pathobiological determinants of atherosclerosis in youth study. J. Am. Med. Assoc. 281:727–735, 1999.
Taylor, C. A., T. J. R. Hughes, and C. K. Zarins. Finite element modeling of three–dimensional pulsatile flow in the abdominal aorta: Relevance to atherosclerosis. Ann. Biomed. Eng. 26:975–987, 1998.
Taylor, C. A., T. J. R. Hughes, and C. K. Zarins. Effect of exercise on hemodynamic conditions in the abdominal aorta. J. Vasc. Surg. 29:1077–1089, 1999.
Wang, J., S. M. Wolin, and H. T. Hintze. Chronic exercise enhances endothelium–mediated dilation of epicardial coronary artery in conscious dogs. Circ. Res. 73:929–1838, 1993.
Wang, K. C., R. W. Dutton, and C. A. Taylor. Geometric image segmentation and image–based model construction for computational hemodynamics. IEEE Eng. Med. Biol. Mag. 18:33–39, 1999.
Womersley, J. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J. Physiol. (London) 127:553–563, 1955.
Zarins, C. K., R. A. Bomberger, and S. Glagov. Local effects of stenoses: Increased flow velocity inhibits atherogenesis. Circulation 64:II–221–227, 1981.
Zarins, C. K., D. P. Giddens, B. K. Bharadvaj, V. S. Sottiurai, R. F. Mabon, and S. Glagov. Carotid bifurcation atherosclerosis: Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 53:502–514, 1983.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, C.A., Cheng, C.P., Espinosa, L.A. et al. In Vivo Quantification of Blood Flow and Wall Shear Stress in the Human Abdominal Aorta During Lower Limb Exercise. Annals of Biomedical Engineering 30, 402–408 (2002). https://doi.org/10.1114/1.1476016
Issue Date:
DOI: https://doi.org/10.1114/1.1476016