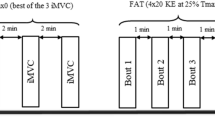
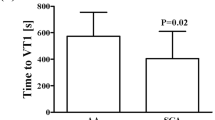
Abstract
Growing evidence suggests that physiological responses during exercise in sickle cell trait (SCT) carriers might differ from persons with normal haemoglobin. Epidemiological and experimental results support the view that SCT carriers could be advantaged in certain anaerobic activities, but the underlying physiological and bio-cellular mechanisms remain unknown. Maximal aerobic physical fitness (i.e. maximal oxygen consumption and maximal aerobic power) is not affected by SCT; however, recent studies suggest that SCT carriers could be characterized by a lesser aerobic capacity. Discrepancies are frequently reported in the literature concerning lactate metabolism during exercise in this population. While some studies observed higher blood lactate concentration during exercise in individuals carrying SCT compared with subjects with normal haemoglobin, others described lower concentration, which suggests a paradoxical lower lactate production by exercising muscles and/or greater ability to clear circulating lactate in SCT carriers. One of the most debated topics is the clinically benign condition of SCT, particularly during strenuous exercise. SCT carriers are usually involved in physical exercise without developing medical complications; however, several authors have presented case reports of SCT carriers who have collapsed and died unexpectedly during or after exercise. Blood rheological, haemostatic and vascular adhesion mechanism abnormalities in combination with environmental factors, such as heat strain, might play a role in the occurrence of these fatal scenarios. Several physiological differences have been observed between SCT carriers and non-SCT carriers, which make it necessary to consider the former as a specific population in response to exercise.
Similar content being viewed by others
References
Korubo-Owiye T. Frequency distribution of haemoglobin genotypes in the Niger-delta of Nigeria. J Expr Clin Sci 1993; 1: 47–50
Mitchell BL. Sickle cell trait and sudden death: bringing it home. J Natl Med Assoc 2007; 99: 300–5
Fabritius H, Millan J, Le Corroller Y. Systematic screening of hemoglobinopathies in blood donors in Guadeloupe(French West Indies) [in French]. Rev Fr TransfusImmunohematol 1978; 21: 937–50
Steinberg MH. Sickle cell trait. In: Steinberg MH, Forget BG, Higgs DR, et al., editors. Disorders ofhemoglobin: genetics, pathophysiology, and clinical management. Cambridge: Cambridge University Press,2001: 811–30
Ashcroft MT, Desai P. Mortality and morbidity in Jamaican adults with sickle-cell trait and with normal hemoglobin followed up for twelve years. Lancet 1976; II: 784–6
Jones SR, Binder RA, Donowho Jr EM. Sudden death in sickle-cell trait. N Engl J Med 1970; 282: 323–5
Ewing PC. Death of an athlete with sickle cell trait. Med World News 1974; 15: 44
Buddington RS, Stahl CJI, McAllister HA, et al. Sports, death, and unusual heart disease [abstract]. Am J Cardiol 1974; 33: 129
Zimmerman J, Granatir R, Mummert K, et al. Sickle crisis precipitated by exercise rhabdomyolysis in apatient with sickle cell trait: case report. Mil Med 1974;139: 313–5
Spencer JD. Case for diagnosis. Mil Med 1980; 145: 396–405
George C. Acute renal failure due to rhabdomyolysis in sickle cell trait. Intensive Care Med 1979; 5: 204–5
Helzlsouer KJ, Hayden FG, Rogol AD. Severe metabolic complications in a cross-country runner with sickle cell trait. JAMA 1983; 249: 777–9
Davis AM. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1988; 318: 787
Kark JA, Posey DM, Schumacher HR, et al. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987; 317: 781–7
Kark JA, Ward FT. Exercise and hemoglobin S. Semin Hematol 1994; 31: 181–225
Dincer HE, Raza T. Compartment syndrome and fatal rhabdomyolysis in sickle cell trait. WMJ 2005; 104: 67–71
Wirthwein DP, Spotswood SD, Barnard JJ, et al. Death due to microvascular occlusion in sickle-cell trait following physical exertion. J Forensic Sci 2001; 46: 399–401
Connes P, Hardy-Dessources MD, Hue O. Counterpoint: sickle cell trait should not be considered asymptomaticand as a begin condition during physical activity. J Appl Physiol 2007; 103: 2138–40
Le Gallais D, Lonsdorfer J, Bogui P, et al. Point: sickle cell trait should be considered asymptomatic and as a begin condition during physical activity. J Appl Physiol 2007; 103: 2137–8
Pearson HA. Sickle cell trait and competitive athletics: is there a risk? Pediatrics 1989; 83: 613–4
Caray AL. Genetic and anthropological studies of Olympic athletes. New York: Academic Press, 1974
Le Gallais D, Lonsdorfer J, Fabritius H, et al. Prevalence of the sickle cell trait among students in a physical education college in Cote-d’Ivoire. Nouv Rev Fr Hematol 1989; 31: 409–12
Thiriet P, Lobe MM, Gweha I, et al. Prevalence of the sickle cell trait in an athletic West African population. Med Sci Sports Exerc 1991; 23: 389–90
Le Gallais D, Prefaut C, Dulat C, et al. Sickle cell trait in Ivory Coast athletic champions, 1956-1989. Int J SportsMed 1991; 12: 509–10
Le Gallais D, Prefaut C, Mercier J, et al. Sickle cell trait as a limiting factor for high-level performance in asemi-marathon. Int J Sports Med 1994; 15: 399–402
Hue O, Julan ME, Blonc S, et al. Alactic anaerobic performance in subjects with sickle cell trait and hemoglobin. Int J Sports Med 2002; 23: 174–7
Connes P, Racinais S, Sara F, et al. Does the pattern of repeated sprint ability differ between sickle cell trait carriers and healthy subjects? Int J Sports Med 2006; 27: 937–42
Connes P, Monchanin G, Perrey S, et al. Oxygen uptake kinetics during heavy submaximal exercise: effect of sickle cell trait with or without alpha-thalassemia. Int J Sports Med 2006; 27: 517–25
Bilé A, Le Gallais D, Mercier B, et al. Anaerobic exercise components during the force-velocity test in sickle cell trait. Int J Sports Med 1996; 17: 254–8
Morrison EY, Cooper PD. Some bio-medical mechanisms in athletic prowess. West Indian Med J 2006; 55: 205–9
Le Gallais D, Lonsdorfer J, Buguet A, et al. frAptitude physique des porteurs du trait drépanocytaire. Sci Sports 1987; 2: 269–77
Bilé A, Le Gallais D, Mercier J, et al. Sickle cell trait in Ivory Coast athletic throw and jump champions, 1956-1995. Int J Sports Med 1998; 19: 215–9
Marlin L, Etienne-Julan M, Le Gallais D, et al. Sickle cell trait in French West Indian elite sprint athletes. Int JSports Med 2005; 26: 622–25
Le Gallais D, Lonsdorfer J, Bogui P, et al. Trait drépanocytaire (AS) et performances en courses de vitesse derésistance et d‘endurance. Sci Sports 1989; 4: 249–51
Green HJ. Muscular adaptations at extreme altitude: metabolic implications during exercise. Int J Sports Med 1992; 13 Suppl. 1: 163–5
Mercier J, Mercier B, Prefaut C. Blood lactate increase during the force velocity exercise test. Int J Sports Med 1991; 12: 17–20
Jones NL, McCartney N, Graham T, et al. Muscle performance and metabolism in maximal isokinetic cycling at slow and fast speeds. J Appl Physiol 1985; 59: 132–6
Davies CT, Young K. Effects of external loading on short term power output in children and young male adults. Eur J Appl Physiol Occup Physiol 1984; 52: 351–4
Bishop D, Spencer M, Duffield R, et al. The validity of a repeated sprint ability test. J Sci Med Sport 2001; 4: 19–29
McGawley K, Bishop D. Reliability of a 5. 6–s maximal cycling repeated-sprint test in trained female team-sportathletes. Eur J Appl Physiol 2006; 98: 383–93
Gozal D, Thiriet P, Mbala E, et al. Effect of different modalities of exercise and recovery on exercise performancein subjects with sickle cell trait. Med Sci Sports Exerc 1992; 24: 1325–31
Murphy JR. Sickle cell hemoglobin (Hb AS) in black football players. JAMA 1973; 225: 981–2
Diggs LW, Flowers E. High school athletes with the sickle cell trait (Hb A/S). J Natl Med Assoc 1976; 68: 492–3
Thiriet P, Le Hesran JY, Wouassi D, et al. Sickle cell trait performance in a prolonged race at high altitude. Med Sci Sports Exerc 1994; 26: 914–8
Embury SH. The interaction of alpha-thalassemia with sickle cell anemia. Hemoglobin 1988; 12: 509–17
Monchanin G, Connes P, Wouassi D, et al. Hemorheology, sickle cell trait, and alpha-thalassemia in athletes: effectsof exercise. Med Sci Sports Exerc 2005; 37: 1086–92
Becklake MR, Griffiths SB, Mc GM, et al. Oxygen dissociation curves in sickle cell anemia and in subjects withthe sickle cell trait. J Clin Invest 1955; 34: 751–5
Reid HL, Obi GO. A study of erythrocyte deformability in sickle cell disease. Trop Geogr Med 1982; 34: 43–6
Ambrus JL, Bannerman RM, Sills RH, et al. Studies on the vasoocclusive crisis of sickle cell disease, III. In vitroand in vivo effect of the pyrimido-pyrimidine derivative,RA-233: studies on its mechanism of action. J Med 1987; 18: 165–98
Obiefuna PC. Rouleaux formation in sickle cell traits. J Trop Med Hyg 1991; 94: 42–4
Brandao MM, Fontes A, Barjas-Castro ML, et al. Optical tweezers for measuring red blood cell elasticity: application to the study of drug response in sickle cell disease. Eur J Haematol 2003; 70: 207–11
Connes P, Sara F, Hardy-Dessources MD, et al. Does higher red blood cell (RBC) lactate transporter activity explain impaired RBC deformability in sickle cell trait? J Physiol 2005; 55: 385–7
Connes P, Sara F, Hardy-Dessources MD, et al. Effects of short supramaximal exercise on hemorheology insickle cell trait carriers. Eur J Appl Physiol 2006; 97 (2): 143–50
McHedlishvili G. Disturbed blood flow structuring as critical factor of hemorheological disorders in microcirculation. Clin Hemorheol Microcirc 1998; 19: 315–25
Parthasarathi K, Lipowsky HH. Capillary recruitment in response to tissue hypoxia and its dependenceon red blood cell deformability. Am J Physiol 1999; 277: 2145–57
Connes P, Yalcin O, Baskurt O, et al. In health and in a normoxic environment,VO2max is/is not limited primarilyby cardiac output and locomotor muscle blood flow[letter]. J Appl Physiol 2006; 100: 2099
Brun JF, Fons C, Supparo C, et al. Could exercise-induced increase in blood viscosity at high shear rate be entirelyexplained by hematocrit and plasma viscosity changes? Clin Hemorheol 1993; 13 (2): 187–99
Brun JF, Khaled S, Raynaud E, et al. The triphasic effects of exercise on blood rheology: which relevance to physiologyand pathophysiology? Clin Hemorheol Microcirc 1998; 19: 89–104
Cresta M. Energy expenditure in sicklemia [letter]. JAMA 1974; 228: 287
Robinson JR, Stone WJ, Asendorf AC. Exercise capacity of black sickle cell trait males. Med Sci Sports 1976; 8: 244–5
Nuss R, Loehr JP, Daberkow E, et al. Cardiopulmonary function in men with sickle cell trait who resideat moderately high altitude. J Lab Clin Med 1993; 122: 382–7
Weisman IM, Zeballos RJ, Martin TW, et al. Effect of army basic training in sickle-cell trait. Arch Intern Med 1988; 148: 1140–4
Weisman IM, Zeballos RJ, Johnson BD. Effect of moderate inspiratory hypoxia on exercise performance in sicklecell trait. Am J Med 1988; 84: 1033–40
Weisman IM, Zeballos RJ, Johnson BD. Cardiopulmonary and gas exchange responses to acute strenuous exerciseat 1,270 meters in sickle cell trait. Am J Med 1988; 84: 377–83
Martin TW, Weisman IM, Zeballos RJ, et al. Exercise and hypoxia increase sickling in venous blood from anexercising limb in individuals with sickle cell trait. Am J Med 1989; 87: 48–56
Hardy RE, Mukherjee S, Hinds JE, et al. Effects of physical stress on complete blood count and venous blood gasprofile of individuals with sickle cell trait. Acta Haematol 1992; 88: 114–9
Sara F, Hardy-Dessources MD, Voltaire B, et al. Lactic response in sickle cell trait carriers in comparison withsubjects with normal hemoglobin. Clin J Sport Med 2003; 13: 96–101
Sara F, Hardy-Dessources MD, Marlin L, et al. Lactate distribution in the blood compartments of sickle cell traitcarriers during incremental exercise and recovery. Int J Sports Med 2006; 27: 436–43
Bilé A, Le Gallais D, Mercier B, et al. Blood lactate concentrations during incremental exercise in subjectswith sickle cell trait. Med Sci Sports Exerc 1998;30: 649–54
Marlin L, Sara F, Antoine-Jonville S, et al. Ventilatory and lactic thresholds in subjects with sickle cell trait. Int J Sports Med 2007; 28: 916–20
Marlin L, Connes P, Antoine-Jonville S, et al. Cardiorespiratory responses during three repeated incrementalexercise tests in sickle cell trait carriers. Eur J Appl Physiol 2008; 102: 181–7
Poiseuille JLM. frRecherches sur les causes du mouvement du sang dans les vaisseaux capillaires. C R Acad Sci Paris 1835; 1: 554–60
Freund H, Lonsdorfer J, Oyono-Enguelle S, et al. Lactate exchange and removal abilities in sickle cell trait carriersduring and after incremental exercise. Int J Sports Med 1995; 16: 428–34
Sara F, Connes P, Hue O, et al. Faster lactate transport across red blood cell membrane in sickle cell trait carriers. J Appl Physiol 2006; 100: 437–42
Lonsdorfer J, Bogui P, Boutros-Toni F, et al. Physiopathologie cardio-respiratoire dans les hémoglobinopathiesà Hb S. Med Trop 1986; 22: 31–46
Ashcroft MT, Desai P, Richardson SA, et al. Growth, behaviour, and educational achievement of Jamaican children with sickle-cell trait. BMJ 1976; 1: 1371–3
Rehan N. Growth status of children with and without sickle cell trait. Clin Pediatr (Phila) 1981; 20: 705–9
Gupta AK, Kirchner KA, Nicholson R, et al. Effects of alpha-thalassemia and sickle polymerization tendency onthe urine-concentrating defect of individuals with sicklecell trait. J Clin Invest 1991; 88: 1963–8
Heller P, Best WR, Nelson RB, et al. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenasedeficiency in hospitalized black male patients. N Engl J Med 1979; 300: 1001–5
Davis Jr CJ, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma: the seventh sickle cell nephropathy. Am J Surg Pathol 1995; 19: 1–11
Sears DA. The morbidity of sickle cell trait: a review of the literature. Am J Med 1978; 64: 1021–36
Miller Jr JM. Sickle cell trait in pregnancy. South Med J 1983; 76: 962–3
Michelson PE, Pfaffenbach D. Retinal arterial occlusion following ocular trauma in youths with sickle-traithemoglobinopathy.Am J Ophthalmol 1972; 74: 494–7
Goldberg MF. The diagnosis and treatment of secondary glaucoma after hyphema in sickle cell patients. Am J Ophthalmol 1979; 87: 43–9
Wolf A, Shalem M, Horowitz J, et al. Retinal vascular occlusion following traumatic hyphema and glaucoma, as a presenting sign of sickle cell trait. Isr Med Assoc J 2005; 7: 476–7
Malhotra V, Prakash R, Choi YS, et al. Fatal pulmonary infarction in a patient with sickle trait. Chest 1973; 64: 524–6
Ebong WW. Avascular necrosis of the femoral head associated with haemoglobinopathy. Trop Geogr Med 1977;29: 19–23
Jadavji T, Prober CG. Dactylitis in a child with sickle cell trait. CMAJ 1985; 132: 814–5
Taylor PW, Thorpe WP, Trueblood MC. Osteonecrosis in sickle cell trait. J Rheumatol 1986; 13: 643–6
Dowling MM. Sickle cell trait is not a risk factor for stroke. Arch Neurol 2005; 62: 1780–1
Golomb MR. Sickle cell trait is a risk factor for early stroke. Arch Neurol 2005; 62: 1778–9
Steen RG, Hankins GM, Xiong X, et al. Prospective brain imaging evaluation of children with sickle cell trait: initial observations. Radiology 2003; 228: 208–15
Connes P, Martin C, Barthelemy JC, et al. Nocturnal autonomic nervous system activity impairment in sickle cell trait carriers. Clin Physiol Funct Imaging 2006; 26: 87–91
Connes P, Hue O, Hardy-Dessources MD, et al. Hemorheology and heart rate variability: is there a relationship? Clin Hemorheol Microcirc 2008; 38: 257–65
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement,physiological interpretation and clinical use. Circulation 1996; 93: 1043–65
Rahimi Z, Merat A, Haghshenass M, et al. Plasma lipids in Iranians with sickle cell disease: hypocholesterolemiain sickle cell anemia and increase of HDL-cholesterol insickle cell trait. Clin Chim Acta 2006; 365: 217–20
Ould Amar AK, Pi Gibert A, Darmon O, et al. AS heterozygote hemoglobinopathy and coronary risk [inFrench]. Arch Mal Coeur Vaiss 1999; 92: 1727–32
Austin H, Key NS, Benson JM, et al. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood 2007; 110: 908–12
Westerman MP, Green D, Gilman-Sachs A, et al. Coagulation changes in individuals with sickle cell trait. Am JHematol 2002; 69: 89–94
Ajayi AA, Kolawole BA. Sickle cell trait and gender influence type 2 diabetic complications in African patients. Eur J Intern Med 2004; 15: 312–5
Bencaiova G, Krafft A, Zimmermann R. Is the sickle cell trait a risk factor in patients with type 2 diabetes mellitus?[letter]. Eur J Intern Med 2005; 16: 462
Reid HL, Oli JM. The possible significance of abnormal blood rheology in diabetics with sickle-cell trait (HbAS). West Afr J Med 1986; 5: 249–56
Oli JM, Reid HL. Do Nigerian diabetics with haemoglobin genotype Hb AS have greater risks of developing renalcomplications and hypertensio4n? A preliminary report. Trop Geogr Med 1985; 37: 309–13
Biedrzycki O, Gillespie H, Lucas S. Sudden death in a patient newly diagnosed with diabetes having hyperosmolarnon-ketotic acidosis with sickle cell trait J Clin Pathol 2006; 59: 882–3
Reid HL, Famodu AA. Spectrophotometric quantitation of haemoglobin S fraction in heterozygous sickle-celltrait (HbAS). Med Lab Sci 1988; 45: 143–5
Baskurt OK, Meiselman HJ, Bergeron MF. Re. point: counterpoint. Sickle cell trait should/should not be consideredasymptomatic and as a benign condition during physical activity. J Appl Physiol 2007; 103 (6): 2142;author reply 2143
Koppes GM, Daly JJ, Coltman Jr CA, et al. Exertioninduced rhabdomyolysis with acute renal failure anddisseminated intravascular coagulation in sickle cell trait. Am J Med 1977; 63: 313–7
Hynd RF, Bharadwaja K, Mitas JA, et al. Rhabdomyolysis, acute renal failure, and disseminated intravascularcoagulation in a man with sickle cell trait. South Med J 1985; 78: 890–1
Sarteriale M, Hart P. Unexpected death in a black military recruit with sickle cell trait: case report. Mil Med 1985;150: 602–5
Rosenthal MA, Parker DJ. Collapse of a young athlete. Ann Emerg Med 1992; 21: 1493–8
Le Gallais D, Bile A, Mercier J, et al. Exercise-induced death in sickle cell trait: role of aging, training,and deconditioning. Med Sci Sports Exerc 1996; 28: 541–4
Kerle KK, Nishimura KD. Exertional collapse and sudden death associated with sickle cell trait. Am Fam Physician 1996; 54: 237–40
Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med 1994; 159: 160–3
Thogmartin JR. Sudden death in police pursuit. J Forensic Sci 1998; 43: 1228–31
NATA. Consensus statement: sickle cell trait and the athlete [online]. Available from URL: http://www.nata.org/statements/consensus/sicklecell.pdf [Accessed 2007Jun 21]
Makaryus JN, Catanzaro JN, Katona KC. Exertional rhabdomyolysis and renal failure in patients with sickle cell trait: is it time to change our approach? Hematology 2007; 12: 349–52
Ajayi AA. Should the sickle cell trait be reclassified as a disease state? Eur J Intern Med 2005; 16: 463
Lucas S. The morbid anatomy of sickle cell disease and sickle cell trait. In: Okpala I, editor. Practical managementof haemoglobinopathies. Oxford: Blackwell Publishing Ltd, 2004
Murray MJ, Evans P. Sudden exertional death in a soldier with sickle cell trait. Mil Med 1996; 161: 303–5
Sherry P. Sickle cell trait and rhabdomyolysis: case report and review of the literature. Mil Med 1990; 155: 59–61
Dudley Jr AW, Waddell CC. Crisis in sickle cell trait. Hum Pathol 1991; 22: 616–8
Charache S. Sudden death in sickle trait. Am J Med 1988; 84: 459–61
Chien S, Usami S, Bertles JF. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest 1970; 49: 623–34
Gordon PA, Breeze GR, Mann JR, et al. Coagulation fibrinolysis in sickle-cell disease. J Clin Pathol 1974; 27: 485–9
Famodu AA. Plasma fibrinolytic activity in sickle cell disease. Trop Geogr Med 1988; 40: 331–3
Nash GB, Boghossian S, Parmar J, et al. Alteration of the mechanical properties of sickle cells by repetitive deoxygenation:role of calcium and the effects of calciumblockers. Br J Haematol 1989; 72: 260–4
Kaul DK, Nagel RL. Sickle cell vasoocclusion: many issues and some answers. Experientia 1993; 49: 5–15
Nsiri B, Gritli N, Bayoudh F, et al. Abnormalities of coagulation and fibrinolysis in homozygous sickle cell disease. Hematol Cell Ther 1996; 38: 279–84
Nsiri B, Gritli N, Mazigh C, et al. Fibrinolytic response to venous occlusion in patients with homozygous sickle celldisease. Hematol Cell Ther 1997; 39: 229–32
Ballas SK, Mohandas N. Sickle red cell microrheology and sickle blood rheology. Microcirculation 2004; 11: 209–25
Stuart MJ, Nagel RL. Sickle-cell disease. Lancet 2004; 364: 1343–60
Okpala I. Leukocyte adhesion and the pathophysiology of sickle cell disease. Curr Opin Hematol 2006; 13: 40–4
Awodu OA, Famodu AA. Haemostatic variables and their relationship to body mass index and blood pressure inadult Nigerians with the sickle cell trait. Clin Hemorheol Microcirc 2007; 36: 89–94
Connes P, Tripette J, Chalabi T, et al. Effects of strenuous exercise on blood coagulation activity in sickle cell traitcarriers. Clin Hemorheol Microcirc 2008; 38: 13–21
Duits AJ, Pieters RC, Saleh AW, et al. Enhanced levels of soluble VCAM-1 in sickle cell patients and their specificincrement during vasoocclusive crisis. Clin Immunol Immunopathol 1996; 81: 96–8
Monchanin G, Serpero LD, Connes P, et al. Effects of a progressive and maximal exercise on plasma levels of adhesion molecules in athletes with sickle cell traitwith or without a-thalassemia. J Appl Physiol 2007; 102: 169–73
Tripette J, Connes P, Hedreville M, et al. Patterns of exercise-related inflammatory response in sickle cell traitcarriers. Br J Sports Med. In press
Tripette J, Hardy-Dessources MD, Sara F, et al. Does repeated and heavy exercise impair blood rheology incarriers of sickle cell trait? Clin J Sport Med 2007; 17: 465–70
Senturk UK, Yalcin O, Gunduz F, et al. Effect of antioxidant vitamin treatment on the time course of hematologicaland hemorheological alterations after an exhaustingexercise episode in human subjects. J Appl Physiol 2005; 98: 1272–9
Bergeron MF, Cannon JG, Hall EL, et al. Erythrocyte sickling during exercise and thermal stress. Clin J Sport Med 2004; 14: 354–6
Acknowledgements
No sources of funding were used in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Connes, P., Reid, H., Hardy-Dessources, MD. et al. Physiological Responses of Sickle Cell Trait Carriers during Exercise. Sports Med 38, 931–946 (2008). https://doi.org/10.2165/00007256-200838110-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200838110-00004