Abstract
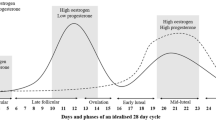
The female hormones, oestrogen and progesterone, fluctuate predictably across the menstrual cycle in naturally cycling eumenorrhoeic women. Other than reproductive function, these hormones influence many other physiological systems, and their action during exercise may have implications for exercise performance. Although a number of studies have found exercise performance — and in particular, endurance performance — to vary between menstrual phases, there is an equal number of such studies reporting no differences. However, a comparison of the increase in the oestrogen concentration (E) relative to progesterone concentration (P) as the E/P ratio (pmol/ nmol) in the luteal phase in these studies reveals that endurance performance may only be improved in the mid-luteal phase compared with the early follicular phase when the E/P ratio is high in the mid-luteal phase. Furthermore, the late follicular phase, characterized by the pre-ovulatory surge in oestrogen and suppressed progesterone concentrations, tends to promote improved performance in a cycling time trial and future studies should include this menstrual phase. Menstrual phase variations in endurance performance may largely be a consequence of changes to exercise metabolism stimulated by the fluctuations in ovarian hormone concentrations. The literature suggests that oestrogen may promote endurance performance by altering carbohydrate, fat and protein metabolism, with progesterone often appearing to act antagonistically. Details of the ovarian hormone influences on the metabolism of these macronutrients are no longer only limited to evidence from animal research and indirect calorimetry but have been verified by substrate kinetics determined with stable tracer methodology in eumenorrhoeic women. This review thoroughly examines the metabolic perturbations induced by the ovarian hormones and, by detailed comparison, proposes reasons for many of the inconsistent reports in menstrual phase comparative research. Often the magnitude of increase in the ovarian hormones between menstrual phases and the E/P ratio appear to be important factors determining an effect on metabolism. However, energy demand and nutritional status may be confounding variables, particularly in carbohydrate metabolism. The review specifically considers how changes in metabolic responses due to the ovarian hormones may influence exercise performance. For example, oestrogen promotes glucose availability and uptake into type I muscle fibres providing the fuel of choice during short duration exercise; an action that can be inhibited by progesterone. A high oestrogen concentration in the luteal phase augments muscle glycogen storage capacity compared with the low oestrogen environment of the early follicular phase. However, following a carbo-loading diet will super-compensate muscle glycogen stores in the early follicular phase to values attained in the luteal phase. Oestrogen concentrations of the luteal phase reduce reliance on muscle glycogen during exercise and although not as yet supported by human tracer studies, oestrogen increases free fatty acid availability and oxidative capacity in exercise, favouring endurance performance. Evidence of oestrogen’s stimulation of 50-AMPactivated protein kinase may explain many of the metabolic actions of oestrogen. However, both oestrogen and progesterone suppress gluconeogenic output during exercise and this may compromise performance in the latter stages of ultra-long events if energy replacement supplements are inadequate. Moreover, supplementing energy intake during exercise with protein may be more relevant when progesterone concentration is elevated compared with menstrual phases favouring a higher relative oestrogen concentration, as progesterone promotes protein catabolism while oestrogen suppresses protein catabolism. Furthermore, prospective research ideas for furthering the understanding of the impact of the menstrual cycle on metabolism and exercise performance are highlighted.



Similar content being viewed by others
References
Reilly T. The menstrual cycle and human performance: an overview. Biol Rhythm Res 2000; 31: 29–40
Lebrun CM. The effect of the phase of the menstrual cycle and the birth control pill on athletic performance. Clin Sports Med 1994; 13: 419–41
Buffenstein R, Poppott SD, McDevitt, RM et al. Food intake and themenstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav 1995; 58: 1067–77
Birch K. Circamensal rhythms in physical performance. Biol Rhythm Res 2000; 31: 1–14
Jurkowski JEH, Jones NL, Toews CJ, et al. Effects of menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J Appl Physiol 1981; 51: 1439–99
Bemben DA, Salm PC, Salm AJ. Ventilatory and blood lactate responses to maximal treadmill exercise during the menstrual cycle. J Sports Med Phys Fitness 1995; 35: 257–62
Dean TM, Perreault L, Mazzeo RS, et al. No effect of menstrual cycle phase on lactate threshold. J Appl Physiol 2003; 95: 2537–43
De Souza MJ, Maguire MS, Rubin K. Effects of menstrual phase and amenorrhea on exercise responses in runners. Med Sci Sports Exerc 1990; 22: 575–80
Beidleman BA, Rock PB, Muza SR, et al. Exercise VE and physical performance at altitude are not affected by menstrual cycle phase. J Appl Physiol 1999; 86: 1519–26
Casazza GA, Suh S-H, Miller BF, et al. Effects of oral contraceptives on peak exercise capacity. J Appl Physiol 2002; 93: 1698–702
Dombovy ML, Bonekat HW, Williams TJ. Exercise performance and ventilatory response in the menstrual cycle. Med Sci Sport Exerc 1987; 19: 111–7
Schoene RB, Robertson HT, Pierson DJ, et al. Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. J Appl Physiol 1981; 50: 1300–5
Smekal G, Von Duvillard SP, Frigo P, et al. Menstrual cycle: no effect on exercise cardiorespiratory variables or blood lactate concentration. Med Sci Sports Exerc 2007; 39 (7): 1098–106
Lebrun CM, McKenzie DC, Prior JC, et al. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc 1995; 27: 437–44
Brutsaert TD, Spielvogel H, Caceres E, et al. Effect of menstrual cycle phase on exercise performance of highaltitude native women. J Exp Biol 2002; 202: 233–9
Stanford KI, Mickleborough TD, Ray S, et al. Influence of menstrual cycle phase on pulmonary function in asthmatic athletes. Eur J Appl Physiol; 96: 703–10
Oosthuyse T, Bosch AN. Influence of menstrual phase on ventilatory responses to submaximal exercise. S Afr J Sports Med 2006; 18: 31–7
Forsyth JJ, Reilly T. The combined effect of time of day and menstrual cycle on lactate threshold. Med Sci Sports Exerc 2005; 37 (12): 2046–53
Zderic TW, Coggan AR, Ruby BC. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol 2001; 90: 447–53
Lavoie J-M, Dionne N, Helie R, et al. Menstrual cycle phase dissociation of blood glucose homeostasis during exercise. J Appl Physiol 1987; 62: 1084–9
McCracken M, Ainsworth B, Hackney AC. Effects of the menstrual cycle phase on the blood lactate response to exercise. Eur J Appl Physiol 1994; 69: 174–5
Campbell SE, Angus DJ, Febbraio MA. Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion. Am J Physiol 2001; 281: E817–25
Nicklas BJ, Hackney AC, Sharp RL. The menstrual cycle and exercise: performance, muscle glycogen, and substrate responses. Int J Sports Med 1989; 10: 264–9
Suh S-H, Casazza GA, Horning MA, et al. Effects of oral contraceptives on glucose flux and substrate oxidation rates during rest and exercise. J Appl Physiol 2002; 93: 42–50
Horton TJ, Miller EK, Glueck D, et al. No effect of menstrual cycle phase on glucose kinetics and fuel oxidation during moderate-intensity exercise. Am J Physiol 2002; 282: E752–62
Brooks-Gunn J, Gargiulo JM, Warren MP. The effect of cycle phase in the performance of adolescent swimmers. Physician Sportsmed 1986; 14: 182–4
Davies BN, Elford JC, Jamieson KF. Variations in performance in simple muscle tests at different phases of the menstrual cycle. J Sports Med Phys Fitness 1991; 31 (4): 532–7
Redman LM, Weatherby RP. Measuring performance during the menstrual cycle: a model using oral contraceptives. Med Sci Sports Exerc 2004; 36 (1): 130–6
Bushman B, Masterson G, Nelsen J. Anaerobic power performance and the menstrual cycle: eumenorrheic and oral contraceptive users. J Sports Med Phys Fitness 2006; 46 (1): 132–7
Middleton LE, Wenger HA. Effects of menstrual phase on performance and recovery in intense intermittent activity. Eur J Appl Physiol 2006; 96: 53–8
Kendrick ZV, Steffen CA, Rumsey WL, et al. Effect of estradiol on tissue glycogen metabolism in exercised oophorectomized rats. J Appl Physiol 1987; 63: 492–6
Jeukendrup A, Saris WHM, Brouns F, et al. A new validated endurance performance test. Med Sci Sports Exerc 1996; 28 (2): 266–70
Bailey SP, Zacher CM, Mittleman KD. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J Appl Physiol 2000; 88: 690–7
McLay RT, Thomson CD, Williams SM, et al. Carbohydrate loading and female endurance athletes: effect of menstrual-cycle phase. Int J Sport Nutr Exerc Metab 2007; 17 (2): 189–205
Oosthuyse T, Bosch AN, Jackson S. Cycling time trial performance during different phases of the menstrual cycle. Eur J Appl Physiol 2005; 94: 268–76
Ruby BC, Robergs RA, Waters DL, et al. Effects of estradiol on substrate turnover during exercise in amenorrheic females. Med Sci Sports Exerc 1997; 29: 1160–9
Devries MC, Hamadeh MJ, Graham TE, et al. 17b-estradiol supplementation decreases glucose rate of appearance and disappearance with no effect on glycogen utilization during moderate intensity exercise in men. J Clin Endo Metab 2005; 90: 6218–25
Palmer GS, Dennis SC, Noakes TD, et al. Assessment of the reproducibility of performance testing on an air-braked cycle ergometer. Int J Sports Med 1996; 17: 293–8
Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol 2002; 282: E1139–46
Campbell SE, Febbraio MA. Effect of ovarian hormones on mitochondrial enzyme activity in fat oxidation pathway of skeletal muscle. Am J Physiol 2001; 281: E803–8
Hatta H, Atomi Y, Shinohara S, et al. The effects of ovarian hormones on glucose and fatty acid oxidation during exercise in female ovariectomized rats. Horm Metab Res 1988; 20: 609–11
D’Eon TM, Sharoff C, Chipkin SR, et al. Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Am J Physiol 2002; 283: E1046–55
Carter SL, McKenzie S, Mourtzakis M, et al. Short-term 17b-estradiol decreases glucose Ra but not whole body metabolism during endurance exercise. J Appl Physiol 2001; 90: 139–46
Devries MC, Mazen JH, Phillips SM, et al. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol 2006; 291: R1120–8
Matute ML, Kalkhoff RK. Sex steroid influence on hepatic gluconeogenesis and glycogen formation. Endocrinology 1973; 92: 762–8
Friedlander AL, Casazza GA, Horning MA, et al. Training- induced alterations of carbohydrate metabolism in women: women respond differently from men. J Appl Physiol 1998; 85 (3): 1175–86
Hackney AC. Influence of oestrogen on muscle glycogen utilization during exercise. Acta Physiol Scand 1999; 167: 273–4
Hackney AC. Effects of the menstrual cycle on resting muscle glycogen content. Horm Metab Res 1990; 22: 647
Tarnopolsky MA, Roy BD, MacDonald JR, et al. Short-term 17-b-estradiol administration does not affect metabolism in young males. Int J Sports Med 2001; 22: 175–80
Shimomura K, Shimizu H, Tsuchiya T, et al. Is leptin a key factor which develops obesity by ovariectomy? Endocr J 2002; 49 (4): 417–23
Rooney TP, Kendrick ZV, Carlson J, et al. Effect of estradiol on the temporal pattern of exercise-induced tissue glycogen depletion in male rats. J Appl Physiol 1993; 75: 1502–6
Beckett T, Tchernof A, Tchernof A. Effect of ovariectomy and estradiol replacement on skeletal muscle enzyme activity in female rats. Metabolism 2002; 51 (11): 1397–401
Latour MG, Shinoda M, Lavoie J-M. Metabolic effects of physical training in ovariectomized and hyperestrogenic rats. J Appl Physiol 2001; 90: 235–41
Van Pelt RE, Gozansky WS, Schwartz RS, et al. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol 2003; 285: E311–7
Hansen PA, McCarthy TJ, Pasia EN, et al. Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats. J Appl Physiol 1996; 80: 1605–11
Cooper BC, Sites CK, Casson PR, et al. Ovarian suppression with a gonadotropin-releasing hormone agonist does not alter insulin-stimulated glucose disposal. Fertil Steril 2007; 87 (5): 1131–8
Toth MJ, Cooper BC, Partley RE, et al. Effect of ovarian suppression with gonadotropin-releasing hormone agonist on glucose disposal and insulin secretion. Am J Physiol 2008; 294: E1035–45
Kalkhoff RK. Metabolic effects of progesterone. Am J Obstet Gynecol 1982; 142: 735–8
Elkind Hirsch KE, Sherman LD, Malinak R. Hormone replacement therapy alters insulin sensitivity in young women with premature ovarian failure. J Clin Endocrinol Metab 1993; 76: 472–5
Ezenwaka EC, Akanji AO, Adejuwon CA, et al. Insulin responses following glucose administration in menstruating women. Int J Gynaecol Obstet 1993; 42: 155–9
Hackney AC, Curley CS, Nicklas BJ. Physiological responses to submaximal exercise at the mid-follicular, ovulatory and mid-luteal phases of the menstrual cycle. Scand J Med Sci Sports 1991; 1: 94–8
Horton TJ, Miller EK, Bourret K. No effect of menstrual cycle phase on glycerol or palmitate kinetics during 90min of moderate exercise. J Appl Physiol 2006; 100: 917–25
Jacobs KA, Casazza GA, Suh S-H, et al. Fatty acid reesterification but not oxidation is increased by oral contraceptive use in women. J Appl Physiol 2005; 98: 1720–31
Wolfe RR. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley-Liss, Incorporated, 1992
Landau BR, Wahren J, Previs SF, et al. Glycerol production and utilization in humans: sites and quantification. Am J Physiol 1996; 271: E1110–7
Elia M, Kahn K, Calder G, et al. Glycerol exchange across the human forearm assessed by a combination of tracer and arteriovenous exchange techniques. Clin Sci 1993; 84: 99–104
Casazza GA, Jacobs KA, Suh S-H, et al. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J Appl Physiol 2004; 97: 302–9
Hellström L, Blaak E, Hagström-Toft E. Gender differences in adrenergic regulation of lipid mobilization during exercise. Int J Sports Med 1996; 17: 439–47
Mittendorfer B, Horowitz JF, Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol 2002; 283: E58–65
Haffner SM, Valdez RA. Endogenous sex hormones: impact on lipids, lipoproteins, and insulin. Am J Med 1995; 98: 40S–7S
Faria ACS, Bekenstein LW, Booth Jr RA, et al. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrin 1992; 36: 591–6
Benoit V, Valette A, Mercier L, et al. Potentiation of epinephrine-induced lipolysis in fat cells from estrogen-treated rats. Biochem Biophys Res Comm 1982; 109: 1186–91
Hansen FM, Fahmy N, Nielsen JH. The influence of sexual hormones on lipogenesis and lipolysis in rat fat cells. Acta Endocrin 1980; 95: 566–70
Ellis GS, Lanza-Jacoby S, Gow A, et al. Effects of estradiol on lipoprotein lipase activity and lipid availability in exercised male rats. J Appl Physiol 1994; 77: 209–15
Spriet LL. Regulation of fat/carbohydrate interaction in human skeletal muscle during exercise. Adv Exp Med Biol 1998; 441: 249–61
Romijn JA, Coyle EF, Sidossis LS, et al. Substrate metabolism during different exercise intensities in endurancetrained women. J Appl Physiol 2000; 88: 1707–14
Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. AmJ Physiol 1993; 265: E380–91
Friedlander AL, Casazza GA, Horning MA, et al. Effects of exercise intensity and training on lipid metabolism in young women. J Appl Physiol 1998; 275: E853–63
Heiling VJ, Jensen MD. Free fatty acid metabolism in the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab 1992; 74: 806–10
Jensen MD, Martin ML, Cryer PE, et al. Effects of estrogen on free fatty acid metabolism in humans. Am J Physiol 1994; 266: E914–20
Magkos F, Patterson BW, Mittendorfer B. No effect of menstrual cycle phase on basal very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Am J Physiol 2006; 291: E1243–9
Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol 2005; 288: E547–55
D’Eon TM, Souza SC, Aronovitz M, et al. Estrogen regulation of adiposity and fuel partitioning: evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 2005; 280 (43): 35983–91
Sidossis LS, Coggan AR, Gastadelli A, et al. A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol 1995; 269: E649–56
Oosthuyse T, Bosch AN, Jackson S. Effect of menstrual phase on the acetate correction factor used in metabolic tracer studies. Can J Appl Physiol 2003; 28: 818–30
Lamont LS, Lemon PWR, Bruot BC. Menstrual cycle and exercise effects on protein catabolism. Med Sci Sports Exerc 1987; 19: 106–10
White LJ, Ferguson MA, McCoy S, et al. Intramyocellular lipid changes in men and women during aerobic exercise: a 1H-magnetic resonance spectroscopy study. J Clin Endocrinol Metab 2003; 88: 5638–43
Tarnopolsky MA, Rennie CD, Robertshaw HA, et al. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol 2007; 292 (3): R1271–8
Devries MC, Lowther SA, Glover AW, et al. IMCL area density, but not IMCL utilization, is higher in women during moderate-intensity endurance exercise, compared with men. Am J Physiol 2007; 293 (6): R2336–42
Zehnder M, Ith M, Kreis R, et al. Gender-specific usage of intramyocellular lipids and glycogen during exercise. Med Sci Sports Exerc 2005; 37 (9): 1517–24
Forsberg AM, Nilsson E, Werneman J, et al. Muscle composition in relation to age and sex. Clin Sci 1991; 81: 249–56
Roepstorff C, Steffensen CH, Madsen M, et al. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. AmJ Physiol 2002; 282: E435–47
Roepstorff C, Donsmark M, Thiele M, et al. Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am J Physiol 2006; 291: E1106–14
Steffensen CH, Roepstorff C, Madsen M, et al. Myocellular triacylglycerol breakdown in females but not in males during exercise. Am J Physiol 2002; 282: E634–42
Jrgensen SB, Richter EA, Wojtaszewski JFP. Role of AMPK in skeletal muscle metabolism regulation and adaptation in relation to exercise. J Physiol 2006; 574 (1): 17–31
Steinberg GR, Macaulay SL, Febbraio MA, et al. AMP activated protein kinase: the fat controller of the energy railroad. Can J Physiol Pharmacol 2006; 84: 655–65
Roepstorff C, Thiele M, Hillig T, et al. Higher skeletal muscle a2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol 2006; 574 (1): 125–38
Wiik A, Gustafsson T, Esbjo rnssonM, et al. Expression of oestrogen receptor alpha and beta is higher in skeletal muscle of highly endurance-trained than of moderately active men. Acta Physiol Scand 2005; 184 (2): 105–12
Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 2003; 52: 268–76
Kleiblova P, Springer D, Haluzik M. The influence of hormonal changes during menstrual cycle on serum adiponectin concentration in healthy women. Physiol Res 2006; 55: 661–6
Kriengsinyos W, Wykes LJ, Goonewardene LA, et al. Phase of menstrual cycle affects lysine requirement in healthy women. Am J Physiol 2004; 287: E489–96
Lariviere F, Moussalli R, Garrel DR. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am J Physiol 1994; 267: E422–8
Toth MJ, Sites CK, Matthews DE, et al. Ovarian suppression with gonadotropin-releasing hormone agonist reduces whole body protein turnover in women. Am J Physiol 2006; 291: E483–90
Hamadeh MJ, Devries MC, Tarnopolsky MA. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J Clin Endocrinol Metab 2005; 90 (6): 3592–9
Miller BF, Hansen M, Olesen JL, et al. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol 2006; 290: E163–8
Acknowledgements
Studies cited in this review as unpublished findings were supported by grants from the University of the Witwatersrand Research Council, Medical Research Council of South Africa and the National Research Foundation. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oosthuyse, T., Bosch, A.N. The Effect of the Menstrual Cycle on Exercise Metabolism. Sports Med 40, 207–227 (2010). https://doi.org/10.2165/11317090-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11317090-000000000-00000