Abstract
Introduction: Alzheimer’s disease (AD) is common among the elderly; it is responsible for 60–80% of all dementia cases. AD is characterized by cognitive decline, behavioural and psychological symptoms, and reductions in functioning and independence. Because of its progressive neurodegenerative nature and unknown aetiology, the burden of AD becomes increasingly significant in an aging population. Estimates indicate that 35.6 million people worldwide suffered from AD in 2010. By 2030 and 2050, this figure is predicted to increase to 65.7 million and 115.4 million, respectively. Costs will also rise along with the increase in the number of people diagnosed with AD. In 2010, the world-wide costs associated with dementia were estimated to be $US604 billion.
Objective: The objective of this study was to conduct a systematic review of current publications dealing with the pharmacoeconomic factors associated with AD medications and to describe the decision-analytic models used to evaluate long-term outcomes.
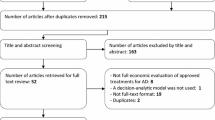
Methods: A systematic literature search was performed to identify articles published between 1 January 2007 and 15 July 2010. The search was also based on a previous systematic review, which included literature up to 2007. Articles were included if they were complete and original economic evaluations of AD and if they were comparative in nature. A quality assessment of the included publications was conducted and relevant information was extracted into tables.
Results: Seven out of 2067 identified articles were included in this systematic review. Four articles evaluated treatment with donepezil, one with galantamine and two with memantine. The studies were conducted in America, Europe and Asia.
Five different groups of medications were compared. The incremental cost-effectiveness ratios (ICERs) for the group of patients treated with donepezil versus no drug treatment ranged from a dominant value to h281416.13 per quality-adjusted life-year (QALY). Patients treated with donepezil versus placebo showed ICERs with a range from a dominant value (not specified) up to h20 866.77 per QALY. Treatment with memantine in addition to donepezil versus treatment with donepezil alone showed an ICER range from a dominant value to €6818.33 per QALY. In comparison with the memantine treatment as an add-on therapy, the ICER of memantine monotherapy versus standard care (without cholinesterase inhibitors [CEIs]) ranged from a dominant value to €63 087.20 per QALY. Finally, the economic evaluation of galantamine in comparison with usual care without any AD drugs showed ICERs ranging from h1894.70 to h6953 per QALY.
Conclusion: The seven identified publications included in this review indicate that treatment with CEIs or memantine seems to be reasonable in terms of clinical effects and costs for patients with AD. Depending on different hypotheses, assumptions and variables (e.g. time horizon, discount rates, initial number of patients in different states, etc.) in the sensitivity analyses, treatment with these drugs seems to be primarily a cost-effective strategy or even a cost-saving strategy. Nevertheless, the results generally are associated with a degree of uncertainty. The comparability of the results from the different economic evaluations is limited because of the different assumptions made.





Similar content being viewed by others
References
Wimo A, Prince M. Alzheimer’s Disease International. World Alzheimer report 2010: the global economic impact of dementia [online]. Available from URL: http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf [Accessed 2010 Oct 22]
Ferri C, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366(9503): 2112–7
Agüero-Torres H, Fratiglioni L, Winblad B. Natural history of Alzheimer’s disease and other dementias: review of the literature in the light of the findings from the Kungsholmen Project. Int J Geriatr Psychiatry 1998 Nov; 13(11): 755–66
Jones R, McCrone P, Guilhaume C. Cost effectiveness of memantine in Alzheimer’s disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Aging 2004; 21(9): 607–20
Klafki H, Staufenbiel M, Kornhuber J, et al. Therapeutic approaches to Alzheimer’s disease. Brain 2006 Nov; 129(11): 2840–55
Mangialasche F, Solomon A, Winblad B, et al. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol 2010 Jul; 9(7): 702–16
Blennow K, de Leon M, Zetterberg H. Alzheimer’s disease. Lancet 2006 Jul; 368(9533): 387–403
Jonsson L, Wimo A. The cost of dementia in Europe: a review of the evidence, and methodological considerations. Pharmacoeconomics 2009; 27(5): 391–403
Quentin W, Riedel-Heller S, Luppa M, et al. Cost-of-illness studies of dementia: a systematic review focusing on stage dependency of costs. Acta Psychiatr Scand 2010 Apr; 121(4): 243–59
Oremus M, Aquilar S. A systematic review to assess the policy-making relevance of dementia cost-of-illness studies in the US and Canada. Pharmacoeconomics 2011 Feb; 29(2): 141–56
Oremus M. Systematic review of economic evaluations of Alzheimer’s disease medications. Expert Rev Pharmacoecon Outcomes Res 2008 Jun; 8(3): 273–89
Fuh J, Wang S. Cost-effectiveness analysis of donepezil for mild to moderate Alzheimer’s disease in Taiwan. Int J Geriatr Psychiatry 2008 Jan; 23(1): 73–8
Gagnon M, Rive B, Hux M, et al. Cost-effectiveness of memantine compared with standard care in moderate-to-severe Alzheimer disease in Canada. Can J Psychiatry 2007 Aug; 52(8): 519–26
Getsios D, Blume S, Ishak K, et al. Cost effectiveness of donepezil in the treatment of mild to moderate Alzheimer’s disease: a UK evaluation using discrete-event simulation. Pharmacoeconomics 2010 May; 28(5): 411–27
López-Bastida J, Hart W, Garcia-Perez L, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. J Alzheimers Dis 2009; 16(2): 399–407
Suh G-H. Modeling the cost-effectiveness of galantamine for mild to moderately severe Alzheimer’s disease in Korea. Value Health 2009 Nov–Dec; 12 Suppl. 3: S49–54
Teipel SJ, Ewers M, Reisig V, et al. Long-term cost-effectiveness of donepezil for the treatment of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 2007 Sep; 257(6): 330–6
Weycker D, Taneja C, Edelsberg J, et al. Cost-effectiveness of memantine in moderate-to-severe Alzheimer’s disease patients receiving donepezil. Curr Med Res Opin 2007 May; 23(5): 1187–97
Neumann PJ. Health utilities in Alzheimer’s disease and implications for cost-effectiveness analysis. Pharmacoeconomics 2005; 23(6): 537–41
National Institute for Health and Clinical Excellence. Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer’s disease: review of NICE. London: NICE, 2006
Neumann PJ, Hermann RC, Kuntz KM, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology 1999; 52: 1138–45
Claxton K, Neumann PJ, Araki S, et al. Bayesian value-of-information analysis: an application to a policy model of Alzheimer’s disease. Int J Technol Assess Health Care 2001; 17: 38–55
Green C, Picot J, Loveman E, et al. Modelling the cost effectiveness of cholinesterase inhibitors in the management of mild to moderately severe Alzheimer’s disease. Pharmacoeconomics 2005; 23(12): 1271–82
Jönsson L, Lindgren P, Wimo A, et al. The cost-effectiveness of donepezil therapy in Swedish patients with Alzheimer’s disease: a Markov model. Clin Ther 1999; 21: 1230–40
Drummond M, Sculpher M, Torrance G, et al. Methods for the economic evaluation of health care programs. 3rd rev. ed. New York: Oxford University Press, 2005
Gold M, Siegel J, Russell L, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996
Weinstein M, Siegel J, Gold M, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996 Oct; 276(15): 1253–8
Leidl R, von der Schulenburg J, Wasem J. Ansätze und Methoden der ökonomischen Evaluation-eine internationale Perspektive. Baden Baden: Nomos Verlagsgesellschaft, 1999
Organisation for Economic Co-operation and Development. OECD.StatExtracts. Purchasing power parity [database]. OECD, 2010 [online]. Available from URL: http://stats.oecd.org/Index.aspx [Accessed 2010 Oct 22]
Preise. Verbraucherpreisindizes für Deutschland. Jahresbericht (Januar 1991–Dezember 2010) [database]. Statistisches Wiesbaden: Bundesamt, 2011 [online]. Available from URL: http://www.destatis.de/jetspeed/portal/cms/Sites/destatis/Internet/DE/Content/Publikationen/Fachveroeffentlichungen/Preise/Verbraucherpreise/VerbraucherpreisindexJahresbericht5611104107004,property=file.pdf [Accessed 2010 Oct 22]
Gustavsson A, van der Putt R, Jönsson L, et al. Economic evaluation of cholinesterase inhibitor therapy for dementia: comparison of Alzheimer’s disease and dementia with Lewy bodies. Int J Geriatr Psychiatry 2009 Oct; 24(10): 1072–8
Kasuya M, Meguro K. Health economic effect of donepezil treatment for CDR 0.5 converters to Alzheimer’s disease as shown by the Markov model. Arch Gerontol Geriatr 2010 May–Jun; 50(3): 295–9
Kirchbach S, Simpson K, Nietert PJ, et al. A markov model of the cost effectiveness of olanzapine treatment for agitation and psychosis in Alzheimer’s disease. Clin Drug Investig 2008; 28(5): 291–303
Wong CL, Bansback N, Lee PE, et al. Cost-effectiveness: cholinesterase inhibitors and memantine in vascular dementia. Can J Neurol Sci 2009 Nov; 36(6): 735–9
National Institute for Health and Clinical Excellence. Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease: review of NICE technology appraisal guidance 111. London: NICE, 2011
Weyerer S. Gesundheitsberichterstattung des Bundes. Altersdemenz. Berlin: Robert Koch-Institut, 2005
Wolfson C, Wolfson D, Asgharian M, et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med 2001 Apr; 344(15): 1111–6
Weinstein M, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices. Modeling studies. Value Health 2003 Jan–Feb; 6(1): 9–17
Drummond M, McGuire A. Economic evaluation in health care: merging theory with practice. New York: Oxford University Press, 2001
Siebert U, Behrend C, Mühlberger N, et al. Entwicklung eines Kriterienkataloges zur Beschreibung und Bewertung ökonomischer Evaluationsstudien in Deutschland. In: Leidl R, von der Schulenburg J, Wasem J, editors. Ansätze und Methoden der ökonomischen Evaluation-eine internationale Perspektive. Baden-Baden: Nomos Verlagsgesellschaft, 1999: 156–70
Acknowledgements
The first and second authors contributed equally to this work. No sources of funding were used in the preparation of this review. The authors of this review have no conflicts of interest that are directly relevant to the content of this review. R.D. has received funding for lectures that were sponsored by pharmaceutical companies that sell CEIs. A full conflict of interest disclosure statement for R.D. is available as Supplemental Digital Content (http://links.adisonline.com/DGZ/A10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouryamout, L., Dams, J., Wasem, J. et al. Economic Evaluation of Treatment Options in Patients with Alzheimer’s Disease. Drugs 72, 789–802 (2012). https://doi.org/10.2165/11631830-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11631830-000000000-00000