Article Text
Abstract
Achilles tendinopathy is prevalent and potentially incapacitating in athletes involved in running sports. It is a degenerative, not an inflammatory, condition. Most patients respond to conservative measures if the condition is recognised early. Surgery usually involves removal of adhesions and degenerated areas and decompression of the tendon by tenotomy or measures that influence the local circulation.
- Achilles tendon
- tendinopathy
- tendinosis
- tendinitis
- MRI, magnetic resonance imaging
- US, ultrasonography
Statistics from Altmetric.com
In the past three decades, the incidence of overuse injury in sports has risen enormously,1 not only because of the greater participation in recreational and competitive sporting activities, but also as a result of the increased duration and intensity of training.2,3 Excessive repetitive overload of the Achilles tendon is regarded as the main pathological stimulus that leads to tendinopathy.4 Kvist2 reviewed 455 athletes with Achilles tendon problems. He found that 53% of them were involved in running sports and 11% were soccer players, emphasising the aetiological role of running. The rest of the patients were involved in other sports in which running was an important training means.
Achilles tendinopathy is not always associated with excessive physical activity, and in a series of 58 Achilles tendinopathy patients, 31% did not participate in sports or vigorous physical activity.5 Also, the use of quinolone antibiotics is associated with Achilles tendinopathy and rupture.6–,8
In this review, we concentrate on tendinopathy of the main body of the Achilles tendon. We shall not deal with Haglund's condition, insertional tendinopathy, or lesions of the myotendinous junction.
METHOD
A computerised literature search of the entire Medline database, covering the years 1966 to the present, was conducted for this review. Table 1⇓ lists the keywords used in the search. All articles relevant to the subject were retrieved, either locally or by interlibrary loan. The search was not limited to the English literature, and articles in all journals were considered. The authors' own personal collections of papers and any relevant personal correspondence were also included. The references selected were reviewed by the authors, and judged on their contribution to the body of knowledge of this topic. The conduct and validity of any clinical studies was carefully considered, and the outcomes of management protocols were carefully scrutinised. Case reports were excluded, unless they mentioned a specific association with the condition that was thought to be relevant to the discussion. Only papers that made a significant contribution to the understanding of this condition were included in the review. This left a total of 347 publications, of which 135 were directly related to the topic of this review.
Keywords used to search the Medline database
ANATOMY OF THE ACHILLES TENDON
The gastrocnemius muscle merges with the soleus to form the Achilles tendon in two different ways. In the more common type 1 junction, the two aponeuroses join 12 cm proximal to their calcaneal insertion. In type 2, the gastrocnemius aponeurosis inserts directly into the aponeurosis of the soleus.9 The Achilles tendon has a round upper part and is relatively flat in its distal 4 cm.10 The fibres of the Achilles tendon are not vertical, but spiral 90°. This arrangement increases the tendon elongation and helps the release during locomotion of the energy stored within the tendon.11,12
Unlike other tendons around the ankle, which have a synovial sheath, the Achilles tendon is enveloped by a paratenon, a membrane consisting of two layers: a deeper layer surrounding and in direct contact with the epitenon, and a superficial layer, the peritenon,13,14 which is connected with the underlying layer through the mesotenon. The paratenon originates from the deep fascia of the leg, the fascia cruris, covering the tendon posteriorly. Recently an organised thickening of the paratenon has been described as the “watershed band”, consisting of a thickened portion of the paratenon from the deep fascia of the posterior aspect of the leg to envelope the watershed region in the Achilles tendon.15
A microvascular perfusion study in the human Achilles tendon assessed by laser Doppler flowmetry showed that the blood flow was considerably lower near the calcaneal insertion but otherwise was distributed evenly in the tendon.16 Further, blood flow in the symptomatic Achilles tendinopathy was considerably elevated compared with the control tendons.17
Langberg et al18 measured blood flow in the peritendinous space of the human Achilles tendon at rest and after 40 minutes of dynamic contraction of the triceps surae. Blood flow in the peritendinous space 5 cm proximal to its insertion increased fourfold with exercise, while it increased only 2.5-fold when measured 2 cm proximal to the insertion.18 The increase in blood flow during exercise probably results from the considerable rise in the negative tissue pressure in the peritendinous space.19
HISTOLOGY OF NORMAL ACHILLES TENDON
Tenocytes and tenoblasts comprise 90–95% of the cellular elements of the tendon. Chondrocytes, vascular cells, synovial cells, and smooth muscle cells form the remaining cellular elements. The extracellular tendon matrix is composed of collagen and elastin fibres, ground substance such as proteoglycans, and organic components such as calcium.20,21
Collagen fibrils are bundled into fascicles containing blood, lymphatic vessels, and nerves,22 and have been shown to interchange between fascicles.23 The fascicles, which are surrounded by the endotenon, group together to form the gross structure of the tendon. The tendon is enveloped by a well defined layer of connective tissue, the epitenon. This, in its turn, is surrounded by the paratenon, with a thin layer of fluid in between to reduce friction during tendon motion. The innermost layer of the epitenon is in direct contact with the endotenon.
BIOMECHANICS OF THE ACHILLES TENDON
Actin and myosin are present in tenocytes,24 and tendons have almost ideal mechanical properties for the transmission of force from muscle to bone. Tendons are stiff and resilient, with high tensile strength: they can stretch up to 4% before damage.20,25 Achilles tendons in men have higher maximum rupture force and stiffness with a larger cross sectional area than in women, while younger tendons have significantly higher tensile rupture stress and lower stiffness.26
The peak Achilles tendon force and the mechanical work by the calf muscles is 2233 N and 34 J in the squat jump, 1895 N and 27 J in the counter movement jump, and 3786 N and 51 J when hopping.27 The indirect estimation of peak load on the Achilles tendon, normalised to subject body weight, is 6.1–8.2 × body weight during running, with a tensile force of more than 3 kN.28
The loads imposed on the Achilles tendon were measured using a “buckle”-type transducer implanted in the Achilles tendon under local anaesthesia. They reached up to 9 kN during running, corresponding to 12.5 times the body weight, 2.6 kN during slow walking, and less than 1 kN during cycling.12,29–,32
A tendon loses its wavy configuration when it is stretched more than 2%. As collagen fibres deform, they respond linearly to increasing tendon loads.20,33 The normal wavy appearance of the tendon is regained if the strain placed on it remains at less than 4%. At strain levels greater than 8%, macroscopic rupture will occur.25,34,35
CAUSES OF ACHILLES TENDINOPATHY
The causes of Achilles tendinopathy remain unclear.2,4 Various theories link tendinopathies to overuse stresses, poor vascularity, lack of flexibility, genetic make up, sex, and endocrine or metabolic factors (table 2⇓).36
Possible causes of Achilles tendinopathy
Excessive loading of the tendon during vigorous training activities is regarded as the main pathological stimulus.2,4,37 The Achilles tendon may respond to repetitive overload beyond physiological threshold by either inflammation of its sheath or degeneration of its body, or by a combination of the two.38 Damage to the tendon can occur even if it is stressed within its physiological limits when the frequent cumulative microtrauma applied do not leave enough time for repair.1 Microtrauma can result from non-uniform stress within the Achilles tendon from different individual force contributions of the gastrocnemius and soleus, producing abnormal load concentrations within the tendon and frictional forces between the fibrils, with localised fibre damage.39
Tendinopathy has been attributed to a variety of intrinsic and extrinsic factors. Tendon vascularity, gastrocnemius-soleus dysfunction, age, sex, body weight and height, pes cavus deformity, and lateral ankle instability are common intrinsic factors. Excessive motion of the hindfoot in the frontal plane, especially a lateral heel strike with excessive compensatory pronation, is thought to cause a “whipping action” on the Achilles tendon, and predispose it to tendinopathy.40 Also, an appreciable forefoot varus has often been found in patients with Achilles tendon problems.41 Perhaps for these reasons foot orthoses are advocated to control symptoms in chronic Achilles tendinopathy,42 although the scientific evidence from randomised controlled trials for their use is still lacking. Changes in training pattern, poor technique, previous injuries, footwear, and environmental factors such as training on hard, slippery, or slanting surfaces are extrinsic factors that may predispose the athlete to Achilles tendinopathy (table 3⇓).4,37,43–,45 It should be emphasised, however, that these are aetiopathogenetic theories, and a cause-effect relation has not been shown in longitudinal studies based on hypothesis testing.
Possible mechanical causes of Achilles tendinopathy
The general pattern of intratendinous degeneration is common to the ruptured and tendinopathic tendons, but there is a greater degree of degeneration in the ruptured tendons. It is therefore possible that there is a common, as yet unidentified, pathological mechanism that has acted on the tendons, causing tendinosis46 and the clinical picture of tendinopathy.
Recently, changes in expression of genes important in cell-cell and cell-matrix interactions in Achilles tendinopathy have been reported, with downregulation of metalloprotease 3 mRNA in tendinopathic Achilles tendon samples. Levels of type I and type III collagen mRNAs were significantly higher in the tendinopathic samples than “normal”. Therefore, metalloprotease 3 may play an important part in the regulation of tendon extracellular matrix degradation and tissue remodelling.47
There appears to be little biochemical evidence of inflammation in degenerative tendon tissue. With the use of in vivo microdialysis, intratendinous measurements showed that glutamate levels were elevated in painful, degenerative tendon. There was no increase in inflammatory prostaglandin E2 .48,49
PATHOPHYSIOLOGY AND NOMENCLATURE
The pathological label “tendinosis” has been in use for more than two decades to describe collagen degeneration in tendinopathy.50 Despite that, many clinicians still use the term “tendinitis”, implying that the fundamental problem is inflammatory. We advocate the use of the term tendinopathy as a generic descriptor of the clinical conditions in and around tendons arising from overuse.51 The terms tendinosis and tendinitis should be used after histopathological examination.51
Tendinosis is defined by Jozsa and Kannus20 as intratendinous degeneration—that is, hypoxic, mucoid or myxoid, hyaline, fatty, fibrinoid, calcific, or some combination of these—from a variety of causes (ageing, microtrauma, vascular compromise, etc). Histologically, there is non-inflammatory intratendinous collagen degeneration with fibre disorientation and thinning, hypercellularity, scattered vascular ingrowth, and increased interfibrillar glycosaminoglycans20,36,52–,54 (fig 1⇓). Leadbetter53 proposed that tendinosis is a failure of cell matrix adaptation to trauma because of an imbalance between matrix degeneration and synthesis.1,53 Macroscopically, the affected portions of the tendon lose their normal glistening white appearance and become grey and amorphous. The thickening can be diffuse, fusiform, or nodular55 (fig 2⇓).
Histological features of Achilles paratendinopathy in a 33 year old male runner with a 12 month history of tendinopathy resistant to conservative management. Note the disordered appearance, the haphazard evidence of vascular ingrowth, and the hypercellularity. The normal, well ordered collagen fibre appearance has been disrupted. Haematoxylin and eosin; original magnification × 120.
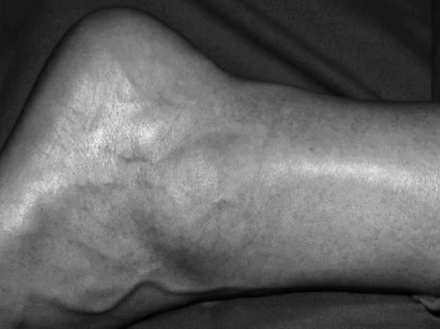
Fusiform swelling at the tendinopathy site of a 38 year old male hockey player with an eight month history of tendinopathy resistant to conservative management. Note the classical location of the swelling.
The paratenon can be involved in the early phases of tendinopathy, and may present as “peritendinitis crepitans” due to adhesion between the tendon and the paratenon. Histologically, tendinosis shows partial disruption in tendon fibres. Tendinosis can be asymptomatic—for example, most patients with an Achilles tendon rupture did not have a clinical picture of tendinopathy before the rupture, and only histology reveals the profound intratendinous changes. Tendinosis may also coexist with symptomatic paratendinopathy.1,44
PAIN IN TENDINOPATHY
Four types of nerve endings can normally be identified in tendons: Ruffini corpuscles; free nerve endings; Pacini corpuscles mainly at the tendon site; the Golgi tendon organs mainly at the muscular site.56 The source of pain in tendinopathy is still under investigation. Classically, pain has been attributed to inflammatory processes, but, as it has become evident that tendinopathies are degenerative not inflammatory conditions, recently the combination of mechanical and biochemical causes has become more attractive.57,58 Tendon degeneration with mechanical breakdown of collagen could theoretically explain the pain mechanism, but clinical and surgical observations challenge this view.58 The biochemical model has become appealing, as many chemical irritants and neurotransmitters may generate pain in tendinopathy. High concentrations of the neurotransmitter glutamate have been found in patients with Achilles tendinopathy.59 The tendons in these patients showed no signs of inflammation, as indicated by the normal prostaglandin E2 levels.59 Substance P and chondroitin sulphate may also be involved in producing pain in tendinopathy.57,58
HEALING PROCESS
The commonest form of tendon healing is by scarring, which is inferior to healing by regeneration.20,53 A tendon heals in essentially the same way as soft tissue, going through the same inflammatory (1–7 days of injury), proliferative (7–21 days), and remodelling (three weeks to one year) phases. Despite collagen maturation and remodelling, tendons are biochemically and metabolically less active than bone and muscle.20,53 Fibroblasts synthesise collagen type III in the proliferative phase. This will be replaced gradually by collagen type I from day 12–14 with progressive increase in tensile strength.20
In animals, by 15 days after surgery, the healing tendons regain 48% tensile strength, 30% of energy absorption, 20% tensile stress, and 14% Young's modulus of elasticity of intact tendons. Healing tendons have 80% of the collagen and 60% of the collagen cross links (hydroxypyridinium) of normal tendons. Healing tendons yield more soluble collagen than intact tendons. This has led to the hypothesis that increased collagen synthesis takes place, possibly with enhanced resorption of mature collagen in healing tendons compared with intact tendons. Electron microscopy shows ultrastructural differences between intact and healing tendons.60
Recovery from tendon injury is slow because of many factors, including low oxygen consumption, slow synthesis of structural protein, and excessive load. The oxygen consumption of tendons is 7.5 times lower than that of skeletal muscles, and tendons are able to sustain loads of up to 17 times body weight.61 Recent studies have shown that the healing capacity of tendons may have been underestimated.62
HISTOLOGY OF ACHILLES TENDINOPATHY
Overuse tendon conditions have traditionally been considered to result from an inflammatory process and treated as such. However, microscopic examination of abnormal tendon tissues shows a non-inflammatory degenerative process.55,63 The present evidence suggests that, in overuse tendinopathy, so-called “tendinitis” is rare. It may occur occasionally in the Achilles tendon in association with a primary tendinosis.
Histopathology of Achilles tendinopathy shows degeneration, a disordered arrangement of collagen fibers, and an increase in vascularity,64–,66 with a singular absence of inflammatory cells and a tendency to poor healing.67 An angioblastic reaction is present, with random orientation of blood vessels, sometimes at right angles to collagen fibres.68 Frank inflammatory lesions and granulation tissue are uncommon and, when found, are associated with tendon ruptures.67
At least six different subcategories of collagen degeneration have been described,69 but Achilles tendon degeneration is usually either “mucoid” or “lipoid”.20 Alcian blue staining shows increased ground substance.20 The characteristic hierarchical structure of collagen fibres is also lost.70,71
Many factors are associated with the pathogenesis of a tendinopathy. These include tissue hypoxia with consequent free radical induced tendon changes caused by ischaemia-reperfusion injury,72 and exercise induced hyperthermia.73 Further, a tendon that has been strained repeatedly to more than 4% of its original length loses elasticity and is at increased risk of a subsequent break in the collagen structure.74 Aged tendons, on the other hand, show little evidence of degeneration. Normal ageing of connective tissue is morphologically different from degeneration.75 Aged tissue has a low rate of metabolism, progressively decreasing elasticity, and low tensile strength.
In 163 patients (75% of whom participated in non-professional sports, particularly running) with classical symptoms and signs of Achilles tendinopathy for a median of 18 months (range three months to 30 years), obvious changes in collagen fibre structure with loss of the normal parallel bundles were evident.76 In those subjects with macroscopic partial ruptures at surgery, fibrin deposits bordered frayed tissue, but histopathology remained identical with those cases without rupture.
This type of Achilles tendon degeneration is evident as increased signal on magnetic resonance imaging (MRI)77–,82 and hypoechoic regions on ultrasound (US).57,80,83,84 These areas of abnormal imaging correspond to areas of altered collagen fibre structure and increased interfibrillar ground substance, which have been shown to consist of hydrophilic glycosaminoglycans.69,84,85
In the paratenon, mucoid degeneration, fibrosis, and vascular proliferation with a slight inflammatory infiltrate only have been reported.10,25,57,86–,98 Astrom and Rausing76 found virtually no evidence of paratenonitis in their series of Achilles tendon specimens. These differences may be explained by the fact that Kvist et al92–,94 did not report pathology of the tendon itself, and studied more active, younger patients. Thus, paratenonitis is not a prerequisite for Achilles tendon symptoms in a population of recreational sportspeople and office workers. The major lesion in chronic Achilles tendinopathy “is a degenerative process characterized by a curious absence of inflammatory cells and a poor healing response”.76 However, the degenerative process is active, with changes in cell activity and phenotype with an increased turnover of matrix and a different expression of genes compared with normal tendons.47
CLINICAL ASPECTS OF ACHILLES TENDINOPATHY
History and examination play a key role in diagnosis and management of Achilles tendinopathy. The onset of pain, duration, and aggravating factors should be documented. Thorough enquiry should be made into the relation of pain to various activities, intensity of training, and exercise technique. Details of previous treatments are also important.
Achilles tendinopathy typically presents with pain 2–6 cm proximal to the tendon insertion after exercise. As the pathological process progresses, pain may occur during exercise, and, in severe cases, the pain interferes with activities of daily living.99 There is good correlation between the severity of the disease and the degree of morning stiffness. Runners experience pain at the beginning and end of a training session, with a period of diminished discomfort in between.100
Clinical examination should start by exposing both legs from above the knees, and the patient should be examined standing and prone. The foot and the heel should be inspected for any malalignment, deformity, obvious asymmetry in tendon size, localised thickening, Haglund heel, and any previous scars. The Achilles tendon should be palpated to detect tenderness, heat, thickening, nodularity, and crepitation.101 The tendon's excursion is assessed. The “painful arc” sign helps to distinguish between tendon and paratenon lesions. In paratendinopathy, the area of maximum thickening and tenderness remains fixed in relation to the malleoli from full dorsiflexion to plantar flexion, whereas lesions within the tendon move with ankle motion.88 Patients with more chronic tendinopathy may have greater difficulty in performing the test than patients who present more acutely,101 although we have not found this test helpful in clinical practice.
IMAGING
US and MRI are the current imaging modalities of choice in patients with Achilles tendinopathy.77,79,102 Historically, in the reports by von Saar103 and Karger,104 radiography provided useful information on the involved Achilles tendon. Although plain soft tissue radiography is no longer the imaging modality of choice in tendon disorders, it still has a role in diagnosing associated or incidental bony abnormalities.
US APPEARANCE OF THE ACHILLES TENDON IN TENDINOPATHY OF THE MAIN BODY
Archambault et al105 used a simple US grading scheme for patients with Achilles tendinopathy: grade 1, normal tendon; grade 2, enlarged tendon; grade 3, a tendon containing a hypoechoic area, regardless of size. The visualised imaged hypoechogenic regions can be nodular, diffuse, or multifocal. The ability to visualise the paratenon and intratendinous areas is dependent on the frequency probes used. Higher frequencies (7.5, 10, 13, and 15 MHz) are more accurate in visualising abnormality lesions in the Achilles tendon main body and paratenon.106
The hypoechoic regions (fig 3⇓) correlate well with macroscopic pathology seen at surgery.77,79,107 However, ultrasonography cannot differentiate partial tendon ruptures from focal degenerative areas.80 Hyperechogenic areas can represent focal accumulation of calcium deposits (fig 4⇓). Movin et al84 used the US guided core biopsy technique to compare the histopathology of biopsy specimens from hypoechoic areas with those from normoechoic areas within Achilles tendon with a clinical diagnosis of tendinopathy. The hypoechoic areas showed a very abnormal tendon structure including an increased amount of proteoglycans. However, moderate pathology was also found in the neighbouring normoechogenous areas within the same tendon, indicating a more generalised disorder than that depicted by US with a 7 MHz transducer.84
Ultrasonographic appearance of Achilles tendinopathy in a 28 year old male soccer player at presentation. The longitudinal scan shows that the tendinopathic tendon is thicker than the asymptomatic contralateral one. The normal, well ordered fibril distribution is lost. Note also the thickening of the paratenon.
Same patient as in fig 2⇑. The transverse scan shows an area of intratendinous calcification. Note the scattered focal hypoechoic areas present over the whole thickness of the tendon.
Clinical value
Patients with tendinopathy of the main body of the Achilles tendon with normal US findings had a shorter time to full recovery than those with enlarged tendons and intratendinous hypoechogenicity.105 Tendon enlargement and alterations in echotexture are risk factors for rupture in patients with Achilles tendinopathy.108
US is routinely used in Europe and is still regarded by many as a primary imaging method for the study of the Achilles tendon, as it correlates well with histopathological findings despite being operator dependent.5,84 The combination of imaging and clinical diagnosis enhances the efficiency of preoperative planning.102,109 US has interactive facilities, which help to reproduce symptoms by transducer compression and concentrate on the pathological area.110
The simultaneous use of colour Doppler with US can allow visualisation of regions of increased vascularity.111 Core biopsies guided by US allow analysis of the pathology of the tendinopathy.112,113 US guided invasive procedures such as percutaneous longitudinal tenotomy can direct management.114
Although US can show alterations in the Achilles tendon with high specificity and sensitivity, it has, like MRI, a high incidence of false positive findings,115 with mild to moderate changes observed in both the involved and uninvolved Achilles tendons not clearly related to the patients' symptoms.116 After surgery, US does not appear to be able to differentiate patients who make a good recovery from those with tendon symptoms.
MRI APPEARANCE OF THE ACHILLES TENDON IN TENDINOPATHY OF THE MAIN BODY
The normal Achilles tendon is usually dark on all imaging sequences.117 In patients with pain located to the main body of the Achilles tendon, MRI may show (a) a thickened paratenon, (b) peritendinous fluid, (c) oedema of Kager's fat pad, (d) thickening of the tendon commonly in a fusiform shape, (e) focal or diffuse intratendinous intermediate or high signal, and (f) interrupted appearances of tendon tissue.78,118
Lesions in the Achilles tendon and in the peritendinous tissues can present in a similar fashion. MRI can help to differentiate between these.78 However, there is significant overlap of MRI findings in symptomatic and asymptomatic Achilles tendons. Furthermore, there is a spectrum of disease in symptomatic tendons, ranging from subtle intratendinous and peritendinous signal to complete tendon tear.119
The normal anatomy of the asymptomatic Achilles tendon is variable, and may be a potential source of diagnostic error.82 An abnormal signal without change in tendon thickness must be interpreted with caution, as the magic angle phenomenon can result in a false positive high signal intensity of normal tendon tissue.120
The sensitivity to depict pathological Achilles tendon tissue can be increased by shortening the echo time121 and by enhancement with a gadolinium contrast agent.84 Under optimal imaging conditions, tendon infrastructure can be evaluated.
Clinical value
MRI can depict the pathology in great detail.78 However, therapeutic guidelines based on MRI are lacking, and its importance in clinical decision making has not been established. The main disadvantage of MRI is its cost, and therefore US has become the primary imaging method in clinical practice in Europe and the Southern hemisphere. Given the high sensitivity of these imaging modalities, an abnormality should be interpreted with caution and correlated with the symptoms before any management recommendations are made.36
Surgical management of chronic Achilles tendinopathy and its healing resulted in a decrease or elimination of the intratendinous signal alteration, including static gadolinium enhanced T1 weighted images, correlating with an improved clinical outcome two years after surgery.81
Shalabi et al81 studied the early dynamic enhancement of the tendon signal with gadolinium contrast agent in patients with Achilles tendinopathy. Early enhancement of the intratendinous signal correlated at histopathology with very abnormal tendon tissue and foci of tenocytes with rounded nuclei. Two years after surgery, the early contrast enhancement of the tendon signal had diminished or disappeared.81
MANAGEMENT
Conservative
Tendinopathy can probably be prevented by encouraging athletes and coaches to follow a sensible training programme.122 Seeking medical attention at an early stage may improve outcome, as treatment becomes more complicated and less predictable when the condition becomes chronic.86,123,124
At present, management of tendinopathy is more an art than a science.36 The efficacy of a conservative rehabilitation programme is debatable. Patients with Achilles tendinopathy (n = 70), with a duration of symptoms of less than six months, were randomised to treatment with either a non-steroid anti-inflammatory drug (piroxicam) or placebo. Both groups received adjunct treatment with a period of rest combined with stretching and strengthening exercises. The overall result after one month was identical, with a rate of success slightly better than 50%.125 Favourable long term prognosis has been reported with a comprehensive conservative protocol that included relative rest, anti-inflammatory drugs, physiotherapy, and orthoses.75,96,116,126,127 Nevertheless, some authors argue that conservative management of chronic Achilles tendinopathy can be time consuming and often unsatisfactory.13
Abstention from the activities that caused the symptoms is recommended in the acute phases. In mild tendinopathy, relative rest or modified activities are prescribed.128 Collagen fibres repair, and remodelling is stimulated by tendon loading. Therefore complete rest of an injured tendon can be counterproductive.
Cyriax97 regarded deep friction massage as a most important technique. In chronic tendinopathy, this should be accompanied by stretching to restore tissue elasticity and reduce the strain in the muscle-tendon unit with joint motion. Augmented soft tissue mobilisation (ASTM) is a new non-invasive soft tissue mobilisation technique which has been successfully used in the treatment of chronic tendinopathy, probably through controlled application of microtrauma.129,130 In a collagenase induced tendinopathy model in rat, light microscopy showed increased fibroblast proliferation with this treatment.129,130
Gentle static stretching by pulling, holding, and releasing the gastrocnemius-soleus complex is the best way of stretching. Another effective way of stretching is by using a wall, stair, or 20° inclined board.44
Eccentric strengthening of the gastrocnemius-soleus muscle and loading of the Achilles tendon are important for both prevention and conservative management of Achilles tendinopathy.131,132 As a rule, gentle strength training should be started early after injury to prevent disuse atrophy, and should not be painful.44
In a prospective multicentre study of 44 patients, with 22 patients (12 men, 10 women; mean age 48 years) in each treatment group, the patients were instructed to perform either eccentric or concentric training on a daily basis for 12 weeks. In both types of treatment regimen, the patients were encouraged to undertake their exercises despite experiencing pain or discomfort in the tendon during exercise. After the eccentric training regimen, 18 of 22 (82%) patients were satisfied and had resumed their previous activity level, compared with eight of 22 (36%) patients who had performed concentric training (p<0.002).
Correction with orthotics can alter the biomechanics of the foot and ankle and relieve heel pain.134,135 Therefore orthotics are commonly used, especially in runners, with up to 75% success.136–,140 A heel lift of 12–15 mm is classically used as an adjunct to the management of Achilles tendon pain .75 However, in a randomised prospective trial of three forms of conservative treatment of sports induced Achilles tendinopathy, the claimed benefit of viscoelastic pads was not substantiated.141
Cryotherapy is used to reduce the metabolic rate of the tendon and to decrease the extravasation of blood and protein from new capillaries found in tendon injuries.142 It also has an analgesic effect.
Therapeutic ultrasound may reduce the swelling in the acute inflammatory phase and experimentally improve tendon healing.98,143,144 Ultrasound also stimulates collagen synthesis in tendon fibroblasts and stimulates cell division during periods of rapid cell proliferation.145
Several drugs, such as low dose heparin, wydase, and aprotinin, have been used in the management of peritendinous and intratendinous pathology.146–,149 Although widely used and promising, evidence of their long term effectiveness is still unclear. Peritendinous injections with corticosteroids are still controversial. One randomised controlled study of 28 patients showed that methyl prednisolone acetate did not improve the cure rate or shorten the healing time in patients with Achilles tendinopathy.150 There is insufficient evidence comparing the risks and benefits of corticosteroid injections in Achilles tendinopathy,151 and some authors believe that steroid injections do not increase Achilles tendon rupture rate.152 Others, however, have shown that intratendinous injections of corticosteroid in animals reduced tendon strength with a potential risk of rupture for several weeks after injection.151–,155
Operative
The natural history of Achilles tendinopathy is still unclear: 24–45.5% of subjects with Achilles tendon problems that fail to respond to conservative management will undergo operative management.13,156 In an eight year longitudinal study of conservative management of patients with Achilles tendinopathy, 24 of the 83 patients (29%) had to be operated on. Seventy patients (84%) had full recovery of their activity level. At eight years, 78 patients (94%) were asymptomatic or had only mild pain with strenuous exercise. However, 34 patients (41%) started to suffer from Achilles tendinopathy in the initially uninvolved contralateral tendon.116,127
Surgery is recommended after exhausting conservative methods of management, often tried for at least six months. However, long standing Achilles tendinopathy is associated with poor results, with a greater rate of reoperation before reaching an acceptable outcome.157 In general, surgical procedures can be broadly grouped into four categories: open tenotomy, with removal of abnormal tissue and the paratenon not stripped; open tenotomy, with removal of abnormal tissue and the paratenon stripped; open tenotomy, with longitudinal tenotomy with or without paratenon stripping; percutaneous longitudinal tenotomy.5,87,158–,160 The objective of surgery is to excise fibrotic adhesions, remove degenerated nodules, and make multiple longitudinal incisions in the tendon to detect intratendinous lesions, restore vascularity, and possibly stimulate the remaining viable cells to initiate cell matrix response and healing.4,5,38,86,96,158 The reasons why multiple longitudinal tenotomies work are still unclear. Recent investigations show that the procedure triggers neoangiogenesis at the Achilles tendon, with increased blood flow.161 This would result in improved nutrition and a more favourable environment for healing.
At surgery, the crural fascia is released on both sides of the tendon. Adhesions around the tendon are then trimmed, and the hypertrophied paratenon is excised.13 In addition, longitudinal splits are made in the tendon to identify the abnormal tendon tissues and excise the areas of degeneration. Reconstruction procedures may be required if large lesions are excised.162
Open operative technique
We perform the operation on an outpatient day case basis. The patient is examined before the operation to correctly identify and mark the area of maximum tenderness and swelling. We normally do not use a tourniquet but lift the end of the operating table 15–20°.163 The patient lies with ankles resting on a sandbag or a pillow and feet hanging over the end of the operating table. A longitudinal slightly curved incision is centred over the abnormal part of the tendon and placed medially, with the concave part toward the tendon. If a lateral approach is used, care should be exerted to avoid the sural nerve and the short saphenous vein.43,164
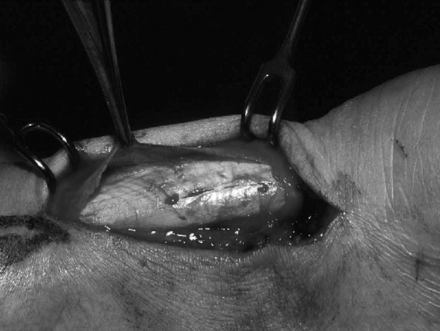
The paratenon and crural fascia are incised and dissected from the underlying tendon. If necessary, the tendon is freed from adhesions on the posterior, medial, and lateral aspects. The paratenon should be excised obliquely, as a transverse excision may produce a constriction ring that may require further surgery.88 The fatty tissue anterior to the tendon should be left intact, as the mesotenon contained within it is an important source of vascular supply to the tendon. Areas of thickened, fibrotic, and inflamed paratenon are excised (fig 5⇓). Inspection of areas lacking normal lustre and careful palpation for thickening, softening, or defects reveals local sections corresponding to areas of tendinosis within the tendon. These zones can be explored with longitudinal tenotomies. The pathology is identified by the change in texture and colour of the tendon. The lesions are then excised, and the defect can either be sutured in a side to side fashion or left open; we normally leave it open. If extensive debridement is required, it is possible to use a turndown flap of the aponeurosis of the medial or lateral head of the gastrocnemius to repair the defect. Occasionally, a tendon transfer may be required if the excision of the degenerated area has left a major defect in the tendon (>50%).165 In most cases, the lesions will be well localised, with normal tendon in between.
Same patient as in fig 4⇑. At operation, a diffusely thickened fibrotic paratenon is seen, with an area of tendon nodularity extending for about 2 cm.
In patients with associated insertional lesions or retrocalcaneal bursitis, an extended approach is used. A full inspection may show an enlarged, inflamed, or scarred retrocalcaneal bursa, adherent to the anterior surface of the Achilles tendon. There may be, in addition, fluid or loose fibrinous bodies within the bursa. After excision of this area, inspection of the posterior superior angle of the calcaneum allows visualisation of any impingement with the insertion of the Achilles tendon with dorsiflexion. This area can be removed with an osteotome, and the sharp edges removed with a rasp. If used, the tourniquet can be deflated, and haemostasis achieved by diathermy. A below knee lightweight cast is applied with the foot plantigrade, and immobilisation is continued for two weeks. Patients are encouraged to weight bear as soon as possible. Greater protection is recommended in patients needing tendon reconstruction. At two weeks, the cast is removed and stretching exercises are started. Sport specific training is started at three months, and competition is resumed at six months.
Experimental operative procedures
Percutaneous longitudinal tenotomy
In patients with isolated Achilles tendinopathy with no paratendinous involvement and a well defined nodular lesion less that 2.5 cm long, we have used multiple percutaneous longitudinal tenotomies when conservative management has failed. A US scan can be used to confirm the precise location of the area of tendinopathy. Patients are mobilised as soon as possible.164,166 If the multiple percutaneous tenotomies are performed in the absence of chronic peritendinopathy, the outcome is comparable to that of open procedures. In addition, it is simple and can be performed in the clinic under local anaesthesia without a tourniquet, but attention to detail is necessary, as even in minimally invasive procedures complications are possible (fig 6⇓).
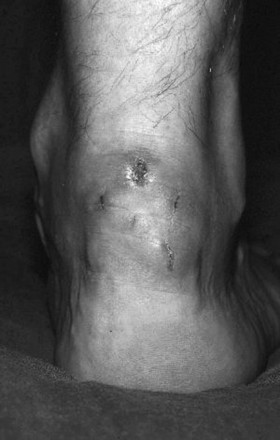
Wound breakdown after open exploration in a 42 year old male fell runner three weeks after the procedure. The patient felt well after removal of the dressing, and went to run. He fell down, and disrupted the wound. Conservative management was elected, and the wound healed over eight weeks. By seven months after the procedure, he was back training.
More recently, we performed US guided percutaneous longitudinal tenotomy under local anaesthesia after failure of conservative management. The procedure had a relatively high rate of success in patients with a single, well defined area of tendinopathy of the main body of the tendon and no paratenon involvement. In patients with diffuse or multinodular tendinopathy or with pantendinopathy, a formal surgical exploration with stripping of the paratenon and multiple longitudinal tenotomies may be preferable.114
Muscle transfer to the body of the tendon
Longitudinal tenotomies increase the blood supply of the degenerated area.161 Recently, in a rabbit model, after longitudinal tenotomy we performed a soleus pedicle graft within the operated tendon, and showed that the transplanted muscle was viable and had integrated well within the tendon tissue three months after the transplant, without transforming into connective tissue. Hypervascularisation of the graft tissue, probably resulting from the operation, was also observed, together with neoangiogenesis up to three months after the operation.64
COMPLICATIONS OF SURGERY
It is remarkable how, for a condition that is relatively common, most studies did not report their assessment procedure, which makes it difficult to compare the results.167 Most authors report excellent or good result in up to 85% of cases, and most articles reporting surgical success rates have over 70% of successful results.46,167 Schepsis and Leach160 report good results in patients with paratendinitis and mucoid degeneration. Kvist44 reports good and excellent results of both paratendinitis and tendinosis. However, this is not always observed in clinical practice.124 In a recent systematic review of the published results of surgery for Achilles tendinopathy, we found an inverse relation between reported success rates and the quality of the scientific methodology used in the study.167 The most common complication of operative management of Achilles tendinopathy is skin breakdown, but deep vein thrombosis and lesions to the sural nerve have been reported.
OUTCOME OF SURGERY
In the most comprehensive study to date, 432 consecutive patients were followed up longitudinally for five months after surgery. If a complication arose, the patient was followed up clinically for at least one year. There were 46 (11%) complications in the 432 patients, and 14 patients with a complication had a reoperation. However, most patients with a complication healed and returned to their previous levels of activity127 (fig 7⇓).
Wound breakdown after percutaneous longitudinal tenotomies in a 55 year old male jogger two weeks after the procedure. Conservative management was instituted, and the wound healed over the following three weeks.
The long term effects of operative management are still not fully clarified. The relative underuse of the affected lower limb after surgery results in prolonged calcaneal bone loss despite early weightbearing loading in patients surgically treated for chronic tendinopathy of the main body of the Achilles tendon. The bone loss had not recovered one year after surgery, but in a comparison group there were no significant side to side differences 39.5 months after the operation.168 Also, the deficit in calf muscle strength seen on the injured side before surgery in this group of patients remained despite them being pain free and active in sports or at recreational level five years after the operation. However, the percentage side to side difference is relatively small, and may not have any clinical relevance.169
METHODS OF EVALUATION
Several quantitative tests of ankle function170 have been used to measure outcome in Achilles tendinopathy. However, condition specific numerical scales generally have greater sensitivity and specificity than do general purpose scales.171 A specific scale for patients with patellar tendinopathy172 has been published, and we have devised a self administered questionnaire based instrument to measure the severity of Achilles tendinopathy, the VISA-A.171 There is a need for a quantitative index of pain and function in patients with Achilles tendinopathy. The VISA-A questionnaire appears to be a valid, reliable, and easy to administer measure of the severity of Achilles tendinopathy, and seems suitable for both clinical rating and quantitative research.
EXPERIMENTAL MODELS OF ACHILLES TENDINOPATHY
Although Achilles tendinopathy is common, experimental models for its study and treatment are uncommon.172–,176 For example, Backman et al172 produced a paratendinopathy with involvement of the main body of the tendon, which showed degenerative changes and increased number of capillaries, by prolonged repeated contractions of the triceps surae (up to six hours per session, three times a week, for up to six weeks) resulting from electrical stimulation producing movements of the ankle joint in anaesthetised rabbits.
In three month old male rats subjected three times a week for one hour to eccentric exercise of one triceps surae muscle (30 stimulations/min) under general anaesthesia, inflammation of the epitenon and paratenon could be induced, but tendon changes corresponding to chronic tendinosis did not develop despite 11 weeks of this regimen.173
More recently, rats have been used to produce acute Achilles tendinopathy by direct trauma.174 In rats, a single intratendinous injection of cytokines produced mild, reversible tendon injury, with no matrix damage or evidence of collagen degradation.176 Again in rats, more prolonged administration of proinflammatory cytokines resulted in diffuse extracellular matrix involvement and collagen fibre derangement and degradation.178
THE FUTURE
Many clinical and biological aspects of Achilles tendinopathy are still unclear. It is classically considered an overuse injury. Nevertheless, some patients seem to be more prone to it than others despite similar training and competition loads. With advances in molecular biology, it may be possible to identify the factors that influence tenocyte metabolism and promote the natural healing process. The role of growth factors in tendon healing is still unclear, although there is evidence that basic fibroblast growth factor can stimulate tendon healing by promoting cell proliferation and matrix synthesis.177 Application of the appropriate growth factors at certain periods during the repair process may improve healing of tendon lesions. However, most of these growth factors are proteins which are rapidly metabolised,178,179 and their delivery is challenging and difficult. Transfer of growth factor genes into tenocytes may eliminate this problem by continuous local release of growth factors at the healing site. Gene transfer for the targeted delivery of growth factors has been used successfully in animal studies,180,181 and transfer of growth factor genes into tenocytes may eliminate this problem by continuous local release of growth factors at the tendinopathy site.182
Take home message
Achilles tendinopathy is prevalent and potentially incapacitating in athletes involved in running sports. It is a degenerative, not an inflammatory, condition, and, until its biology has been elucidated, its management will be based more on empirical than scientific principles.
CONCLUSIONS
Although Achilles tendinopathy has been extensively studied, there is a clear lack of properly conducted scientific research to clarify its causes, pathology, and optimal management plan.
The outcome of Achilles tendinopathy is more favourable when treated within six months of onset. Most patients respond to conservative measures if the condition is recognised early, whereas continuing the offending activities leads to adhesion and chronic changes which are more resistant to conservative treatment. Teaching patients to control the symptoms may be more beneficial than leading them to believe that Achilles tendinopathy is fully curable. Progressive eccentric training has been reported with encouraging short term results.
Surgery usually involves removal of adhesions and degenerated areas and decompression of the tendon by tenotomy or measures that influence the local circulation.
It is still debatable why tendinopathic tendons respond to surgery.58 For example, we do not know whether surgery induces revascularisation, denervation, or both, resulting in pain reduction. It is also unclear how longitudinal tenotomy improves vascularisation.
As the biology of tendinopathy is being clarified, more effective management regimens may come to light, improving the success rate of both conservative and operative management.