Article Text
Abstract
Background: Exercise induced bronchoconstriction (EIB) is common in elite athletes. Eucapnic voluntary hyperventilation (EVH) is a laboratory test recommended for the identification of EIB in athletes, secondary to a field exercise challenge. Montelukast attenuates EIB, but its protective effect against airway narrowing from EVH has not been investigated.
Objective: To examine the effectiveness of montelukast after exercise and after EVH.
Methods: A randomised, placebo controlled, double blind, crossover study was performed with 11 physically active EIB positive subjects (eight men, three women; mean (SD) age 22.8 (6.8) years). Six hours before each of the following challenges 10 mg montelukast or placebo was ingested: (a) a six minute, cold air (−3°C) maximal effort work accumulation cycle ergometer exercise; (b) EVH, breathing 5% CO2 compressed air at 85% maximal voluntary ventilation for six minutes. Spirometry was performed before and 5, 10, and 15 minutes after the challenge. At least 48 hours was observed between challenges.
Results: No differences in forced expiratory volume in one second (FEV1) were found after the two challenges. Exercise and EVH resulted in falls in FEV1 of 22.4 (18.0) and 25.6 (16.8) respectively. Falls in FEV1 after montelukast were less than after placebo (10.6 (10.6) and 14.3 (11.3) after exercise and EVH respectively; p<0.05). Montelukast provided protection against bronchoconstriction (59% and 53%; p<0.05) for eight exercising subjects and 10 EVH subjects; no protection was afforded for three exercising and one EVH challenged subject.
Conclusions: Both exercise and EVH were potent stimuli of airway narrowing. A single dose of montelukast provided reasonable protection in attenuating bronchoconstriction from either exercise or EVH. The similar protection by montelukast suggests that EVH is a suitable laboratory surrogate for EIB evaluation.
- AHR, airway hyper-responsive
- EIB, exercise induced bronchoconstriction
- EVH, eucapnic voluntary hyperventilation
- EX, cold air exercise
- FEF25–75, forced expiratory flow through the mid portion of the vital capacity
- FEV1, forced expiratory volume in one second
- FVC, forced vital capacity
- asthma
- bronchoconstriction
- elite athletes
- leukotrienes
Statistics from Altmetric.com
- AHR, airway hyper-responsive
- EIB, exercise induced bronchoconstriction
- EVH, eucapnic voluntary hyperventilation
- EX, cold air exercise
- FEF25–75, forced expiratory flow through the mid portion of the vital capacity
- FEV1, forced expiratory volume in one second
- FVC, forced vital capacity
Exercise induced bronchoconstriction (EIB) after exercise in elite athletes has received substantial attention over the last decade. EIB is common among elite athletes; about 25% of the 1998 US Winter Olympians were identified by spirometry1 or questionnaire2 as having EIB. More than 15% of the 1996 US Summer Olympians reported a diagnosis of asthma or EIB.3 Self reported symptoms are most often the primary criteria used by clinicians in diagnosing EIB, even though reports show that objective criteria from bronchial provocation tests are needed to make the correct diagnosis in elite athletes.4,5
The high use of β2 agonists among Olympic athletes led the International Olympic Committee-Medical Commission (IOC-MC) to establish objective criteria for allowed use during competition. Inhaled β2 agonists are indicated for prophylaxis of exercise induced asthma or EIB because of the effective relaxation of bronchial smooth muscle. Eucapnic voluntary hyperventilation (EVH) is the laboratory test recommended for the identification of EIB in Olympic athletes,6–9 secondary to a field exercise challenge.10 The EVH challenge involves inhalation of dry air containing 5% CO2 at exercising minute ventilations (VE) for six minutes.5–8,11 The effectiveness of this test is based on the assumption that the resultant airway narrowing from EVH is caused by osmotic events that stimulate the release of bronchoconstricting mediators, similar to the mechanism hypothesised for the response after exercise.12 The ability to standardise and control environmental conditions make this test a suitable alternative to a field based exercise test that can be performed with ease in a clinical or laboratory setting,5,6,8 although a field based exercise challenge in cold dry ambient conditions has been shown to be superior to a laboratory exercise challenge at ambient conditions of 21°C and 50% relative humidity.10
Leukotrienes are involved in the pathogenesis of asthma and EIB by inducing airway smooth muscle contraction.13,14 Montelukast, a long-acting cysteinyl leukotriene receptor antagonist that blocks the action of leukotrienes C4 and D4,15 effectively protects against airway narrowing exercise. Previous reports evaluating the inhibitory effect of montelukast on EIB have identified an approximate 50% improvement in post-exercise forced expiratory volume in one second (FEV1) after treatment.16–20
The aim of this study was to compare airway responses by airway hyper-responsive (AHR) positive subjects to EVH in the laboratory to an exercise challenge in cold/dry air after ingesting either placebo or montelukast. Using montelukast treatment to indirectly evaluate leukotriene involvement in EVH and exercise is important to the elite athlete as (a) EVH has been designated as the preferred challenge by the IOC-MC for obtaining objective evidence for permission of β2 agonist use in Olympic competition and (b) a single dose of montelukast may prove to be an effective treatment modality for EIB.
METHODS
This study was conducted in the spring of 2003. Each subject performed two room temperature EVH challenges and two high intensity six minute cycle ergometer challenges in cold temperature conditions (EX) in the Marywood University Human Performance Laboratory climate control chamber. Subjects ingested a single dose of either 10 mg montelukast or placebo six to eight hours before each trial. The order was randomised and double blinded, and the trials were separated by 48–72 hours. We have previously shown that 48 hours between challenges was sufficient to negate an order effect.8 Spirometry was performed before and after the challenge.
Subjects
After receiving a written and verbal study description, 11 EIB positive (defined by a ⩾10% fall in FEV1) recreational and college athlete subjects (three women, eight men; mean (SD) age, weight, and height 22.8 (6.8) years, 80.5 (15.7) kg, and 173.9 (6.3) cm respectively) volunteered to undergo EVH and EX. The inclusion criteria of a ⩾10% fall in FEV1 is consistent with the IOC-MC recommended cut-off point and was designed to include those with mild to moderate EIB, as this represents the elite athlete population. Four subjects had a previous diagnosis of mild asthma, and were prescribed a short acting β2-agonist, but reported non-compliant use. This study was approved by the Marywood University institutional review board, and all subjects gave written informed consent.
Test conditions
Ambient test conditions for EVH were 21°C and 40% relative humidity. EVH gas was compressed, dry air (21% O2, 5% CO2, balance N2). Environmental conditions for EX in the climate control room were set at −3°C and 50% relative humidity (VWR digital hygrometer/thermometer, VWR International Inc, West Chester, PA, USA). The water content of the inspired air in both challenges made conditions suitable for AHR provocation.12
Pulmonary function tests
Pulmonary function was measured by spirometry using a calibrated computerised pneumotachograph spirometer (Jaeger Masterscope PC, Hoechberg, Germany). Forced vital capacity (FVC), FEV1, FEV1/FVC ratio, and forced expiratory flow through the mid portion of the vital capacity (FEF25–75) were determined before and after the challenge. The procedure for all pulmonary function tests was (a) three normal tidal volume breaths, (b) maximal inhalation, (c) forced maximal exhalation, and (d) maximal inhalation. Resting baseline pulmonary function was established before each challenge by selecting the best of three resting pulmonary function tests based on the highest sum of FVC and FEV1. Pulmonary function after each challenge was measured 5, 10, and 15 minutes after the completion of EVH and EX. If any measurement was technically unacceptable, the pulmonary function test manoeuvre was repeated.
Eucapnic voluntary screening challenge
The EVH protocol required subjects to breathe the compressed EVH gas mixture at a predetermined rate of 85% maximal voluntary ventilation (estimated from 30 × resting FEV1) for six minutes.6,8 Gas flowed from a cylinder through a calibrated rotameter (1110 series flowmeter; Brooks Instruments, Hatfield, Pennsylvania, USA) to a 300 g reservoir bag through high pressure tubing. From the reservoir bag, the gas was directed to the subject through a 35 mm breathing tube, two way breathing valve, and mouthpiece (Hans Rudolf, Kansas City, Missouri, USA). Expired gas passed through a flow sensor, and VE was recorded as a verification of the target VE (VmaxST Measurement Cart, SensorMedics, Yorba Linda, California, USA). Inhaled gas during EVH was at laboratory temperature but completely dry. Ambient conditions in the laboratory were 21°C and 40% relative humidity.
Cycle ergometer exercise challenge
The high intensity EX challenge consisted of a six minute trial using an electronically braked cycle ergometer (Lode Excalibur Sport; Lode, Groningen, the Netherlands). Subjects were instructed to exercise at the highest intensity sustainable for the duration of the test and were verbally encouraged to give a maximal effort. Subjects wore wireless heart rate monitors to verify exercise intensity (Polar Vantage XL; Polar Electro Oy, Kempele, Finland), and total work accumulated (kJ) was recorded on completion of the six minute time trial.
Statistical methods
Descriptive statistics for resting lung function were calculated for each trial (placebo and montelukast) for challenge conditions. Repeated measures analysis of variance was used to analyse differences between trials and groups. An α of p ⩽ 0.05 was considered significant.
RESULTS
Table 1 gives resting baseline lung function values. Mean values for FVC, FEV1, FEV1/FVC, FEF25–75, and peak expiratory flow were within the normative predicted values for age, height, and sex. However, one subject showed <80% predicted FVC, four subjects had <80% predicted FEV1, two subjects had <70% FEV1/FVC, and six subjects had <67% predicted FEF25–75 (of these six, three had FEF25–75 values below 50% predicted). Resting FVC and FEV1 were significantly lower for montelukast than for placebo when values from all four trials were pooled (p<0.05). Within trial modalities (EVH and EX), resting FVC before EX was significantly lower for montelukast than for placebo (p<0.05). No significant correlations were identified between resting lung functions and falls in FEV1 after the challenge for any trial.
Baseline lung functions
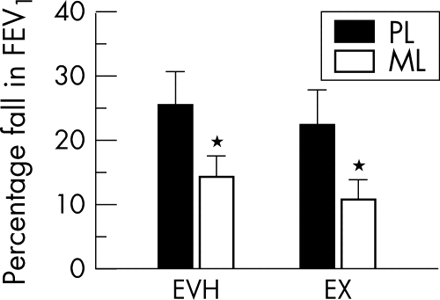
Eleven subjects performed two trials of both EVH and EX for six minutes six to eight hours after ingesting either placebo or montelukast. Percentage peak fall in FEV1 from resting FEV1 between EVH and EX was not different for placebo or for montelukast (25.6 (16.75)% v 22.4 (18.03)% for EVH placebo and EX placebo; 14.3 (11.28)% v 10.6 (10.6)% for EVH montelukast and EX montelukast respectively). Montelukast provided about 44% protection from AHR for EVH and about 53% protection from AHR for EX, expressed as the difference in percentage fall in FEV1 between placebo and montelukast (range 0–100% for EVH and EX, p<0.05; fig 1).
Peak percentage fall in forced expiratory volume in one second (FEV1) from baseline during 15 minutes after eucapnic voluntary hyperventilation (EVH) or high intensity six minute cycle ergometer challenge in cold temperature conditions (EX) after ingestion of placebo (PL) or montelukast (ML). *Significantly different from placebo (p<0.05).
Figure 2 shows change in FEV1 after EVH and EX for placebo and montelukast trials. For EVH, FEV1 at 5, 10, and 15 minutes after the challenge was significantly different between placebo and montelukast (p<0.05); for EX, FEV1 at 10 and 15 minutes was significantly different between placebo and montelukast (p<0.05). No difference was noted for any time point between EVH placebo and EX placebo, or between EVH montelukast and EX montelukast. Likewise, no difference in percentage fall in FEV1 between the 5, 10, and 15 minute time points was identified for any trial. The mean areas above the curves were 267 (195), 137 (118), 212 (196), and 107 (115) for EVH placebo, EVH montelukast, EX placebo, and EX montelukast respectively. Significant differences were noted between placebo and montelukast for both EVH and EX (p<0.05), but not between EVH and EX for the respective placebo and montelukast treatments.
Percentage change from baseline in forced expiratory volume in one second (FEV1) 5, 10, and 15 minutes after the completion of eucapnic voluntary hyperventilation (EVH) and high intensity six minute cycle ergometer challenge in cold temperature conditions (EX) trials on placebo (PL) and montelukast (ML) treatments. *Significant differences between placebo and montelukast for respective challenges at each time point.
Figure 3 presents individual plots for EVH and EX after ingestion of placebo or montelukast. After placebo treatment, 10 subjects were positive to EVH (EVH+), eight were positive to EX (EX+), and seven had concordant findings and were positive to both EVH and EX. Seven subjects showed greater falls in FEV1 from EVH, two showed greater falls from EX, and two had similar falls for EVH and EX. For EVH, all but one subject showed some degree of protection from the single 10 mg dose of montelukast ingested six to eight hours before the challenge. That subject showed 16% and 15.6% falls in FEV1 from EVH for placebo and montelukast respectively, but showed no clinically relevant falls in FEV1 from EX. Three other subjects who tested positive by EVH on placebo and negative by EX on placebo showed no effect from montelukast treatment during EX.
Individual peak percentage falls in forced expiratory volume in one second (FEV1) for eucapnic voluntary hyperventilation (EVH) (A) and high intensity six minute cycle ergometer challenge in cold temperature conditions (EX) (B).
Figure 4 plots the difference between falls in FEV1 after EVH and EX against an estimate of the true value determined as the mean of the two challenges for placebo and montelukast (3.21 (14.51)% and 3.65 (8.84)% for placebo and montelukast respectively); no significant difference was identified between the mean of the differences between the two values. The difference between challenges was normally distributed around the mean of EVH and EX challenges. For placebo, all differences between challenges fell within 2 SD of the mean difference; for montelukast, only one value exceeded 2 SD of the mean, showing reasonable agreement between the hyper-responsiveness to EVH and EX and between the effects of montelukast on the response to both challenges.
Difference in percentage fall in forced expiratory volume in one second (FEV1) between high intensity six minute cycle ergometer challenge in cold temperature conditions (EX) and eucapnic voluntary hyperventilation (EVH) plotted against the mean value for the EX and EVH percentage fall in FEV1 for each subject. All values for placebo fell within 2 SD of the mean and all but one value for montelukast fell within 2 SD of the mean, indicating reasonable agreement between challenges.
DISCUSSION
Airway narrowing in EIB+ subjects after EVH is similar to the transient airway obstruction after exercise in cold/dry air; the small differences between peak fall in FEV1 between EVH and EX for placebo and after a single dose ingestion of montelukast were not significant. EVH identified seven of eight subjects with EIB after EX, with an additional three subjects AHR+ by EVH and AHR− by EX, showing that EVH is a potent stimulus for EIB in people who are hyper-reactive to exercise. These results are similar to our earlier study comparing EVH with a field based exercise challenge where we identified 17 of 19 EIB+ subjects by EVH and only 11 subjects by exercise.8 The similar levels of inhibition of FEV1 from montelukast for EVH and EX suggests that the extent of leukotriene involvement is probably similar in the two challenges. A single 10 mg dose of montelukast provided protection from EIB in ten of 11 subjects after EVH and seven of eight subjects after EX. The ∼50% protection from bronchoconstriction by montelukast for both challenges is in agreement with the degree of protection reported by others for exercise.16–20 This study is the first to compare the effects of montelukast on EIB caused by EVH and exercise. Our findings provide evidence that the modular role of leukotrienes in the bronchoconstrictive response to EVH is probably similar to that observed in response to exercise. Moreover, inhalation of dry air is paramount to the EIB response in most cases.
In this study, a 10 mg dose was taken orally six to eight hours before EVH or EX. As bronchodilation occurs within four hours of montelukast administration14 and peak serum concentration of montelukast has been found to occur four hours after administration,21 we feel that ingestion of montelukast six to eight hours before the challenges in this study was sufficient to be maximally effective.
The post-challenge change in FEV1 was used as a measure of abnormal lung function response and is the most widely accepted index used to define EIB.22,23 FEV1 is the lung function variable accepted by the IOC-MC to identify EIB.9 The 10% fall in FEV1 from the resting value after EVH or EX used to define EIB is consistent with the recommendations of others,8,22–25 and is the cut-off point used to define EIB in athletes requesting approval for β2 agonist use during international competition.6,7,9 The cut-off point is based on the mean value of FEV1 plus 2 SD from the response to an exercise challenge from non-hyper-responsive subjects. Others have statistically justified a more liberal cut-off value for the fall in FEV1 of about 7% in elite athletes.4,26 Although strong correlations have been identified between mid expiratory flow rates (FEF25–75 and FEF50), these values are dependent on vital capacity and need to be interpreted with caution,27 thus we did not report mid expiratory flow rate values. Likewise, we chose not to report peak expiratory flow, as it is highly effort dependent and highly variable.4
Baseline lung function in asthmatics is often associated with hyper-reactive airways. Similar to previous studies using elite athletes,4,8 we did not find a relation between baseline lung function variables and post-challenge falls in FEV1. The mean resting lung function values were considered normal (table 1); however, six subjects showed abnormally low values. Unlike previous findings28 where no change in resting lung function was noted after treatment with montelukast, we found small, but significant decreases in resting FVC and FEV1 when trials for EVH and EX were pooled for placebo and montelukast. As these changes were not significant when EVH and EX baseline functions were analysed separately, clinical relevance is unlikely and they could be due to the small sample size of this study.
What is already known on this topic
It is known that EVH is a potent surrogate challenge for identification of EIB and that inspired dry air is critical to an EIB provoking challenge. It is also known that, overall, montelukast provides about 50% protection against EIB.
What this study adds
This study shows that the contribution of leukotrienes to the post-challenge response to EVH and cold/dry air exercise is equal, suggesting similar mechanisms and supporting EVH as a challenge for EIB. Importantly, this study confirms that a single 10 mg dose of montelukast is effective prophylaxis for EIB.
The precise mediators of EIB depend on the stimulus.29 Not all subjects in this study responded equally to EVH and EX or the montelukast; this is expected in a heterogeneous group. However, analysis of the mean response for EVH and EX and individual differences between the two challenges for both placebo and montelukast indicate normal distribution within 2 SD of the mean. Cold air exercise has been shown to be only partially leukotriene mediated,30–32 the antigen response is highly leukotriene mediated,33 and aspirin induced bronchoconstriction is entirely leukotriene mediated.29,34 Leukotrienes and prostaglandins are generally seen as primary mediators in bronchoconstriction. However, several other mediators may directly or indirectly contribute to the EIB response.12
The partial and equal protection achieved by montelukast for EVH and EX in this study infers that other mediators in addition to leukotrienes are involved in the bronchoconstrictive response from these challenges and that the challenges are similarly mediated. The water content of the EVH air was minimal, as compressed air from a tank contains little water. Our measurement of relative humidity at the inspiratory port during EVH was about 12% at 21°C, and the water content during EX was less than 5 mg per litre of air (−2.66 (0.22)°C and 50% relative humidity). Recommendations of less than 10 mg water per litre of air for an exercise provocation test have been made.6,8,12 Although VE was not measured during EX, it is likely that it was equal to or greater than the VE experienced during EVH, as EX was performed as a maximal effort cycle ergometer ride for six minutes.
In summary, EVH appears to be a reliable laboratory surrogate challenge for cold/dry air exercise as a provocation test for EIB. Although the potency of EVH in identifying airway narrowing is established, this is the first study to directly or indirectly evaluate the role of leukotrienes in both EVH and exercise challenges. Our results indicate that falls in FEV1 are not different between the two challenge modes. The protection afforded by montelukast was similar for EVH and EX, implying that leukotriene mediation of bronchoconstriction after EVH and EX is equal and probably involved in sustaining the airway narrowing rather than initiating it. Given that the preferred challenge for diagnosing EIB for allowed use of β2 agonists during Olympic competition is EVH, this information is important. Equally important is the confirmation that a single 10 mg dose of montelukast can be used as prophylaxis for EIB.
Acknowledgments
We thank the subjects who volunteered for this study. The work was supported by Merck and Co, Inc grant No SING-US-63-01. Montelukast and placebo were a gift from Merck and Co.
REFERENCES
Footnotes
-
Competing interests: none declared