Article Text
Abstract
Objective To determine whether there are objective findings of tendinosis in a rabbit tendinopathy model on exercised and contralateral (non-exercised) Achilles tendons.
Design Four groups of six New Zealand white rabbits per group were used. The animals of one (control) group were not subjected to exercise/stimulation.
Interventions Animals were subjected to a protocol of electrical stimulation and passive flexion–extension of the right triceps surae muscle every second day for 1, 3 or 6 weeks.
Main Outcome Measures Tenocyte number and vascular density were calculated. Morphological evaluations were also performed as well as in-situ hybridisation for vascular endothelial growth factor (VEGF) messenger RNA.
Results There was a significant increase in the tenocyte number after 3 and 6 weeks of exercise, but not after 1 week, in comparison with the control group. This was seen in the Achilles tendons of both legs in experimental animals, including the unexercised limb. The pattern of vascularity showed an increase in the number of tendon blood vessels in rabbits that had exercised for 3 weeks or more, compared with those who had exercised for 1 week or not at all. VEGF-mRNA was detected in the investigated tissue, with the reactions being more clearly detected in the tendon tissue with tendinosis-like changes (6-week rabbits) than in the normal tendon tissue (control rabbits).
Conclusions There were bilateral tendinosis-like changes in the Achilles tendons of rabbits in the current model after 3 weeks of training, suggesting that central neuronal mechanisms may be involved and that the contralateral side is not appropriate as a control.
Statistics from Altmetric.com
Chronic tendon pain, or ‘tendinopathy’, remains a poorly understood condition. Histopathological and biochemical studies of tendinopathic tissues have revealed that chronic tendinopathy is characterised by degenerative-like histopathological tissue changes, referred to collectively as ‘tendinosis’.1 2 These changes include tenocyte hypercellularity, collagen discontinuity, vascular proliferation and apoptosis.3,–,5 The events leading to tendon degeneration and chronic pain have not been fully elucidated.
The insidious onset of tendinosis has made it difficult to recruit research participants during the early stages of the condition, and consequently it is challenging to study the initial phases of pathophysiology. In addition, tissue changes can precede symptoms by many months.6,–,8
Tendon overload or overuse, the most commonly accepted extrinsic risk factors for tendinopathy,2 9,–,11 cannot fully explain the development of the condition, as is clearly demonstrated by the development of Achilles tendinopathy also in inactive individuals.12 13 It remains difficult to reconcile these disparate findings into an evidence-based management approach without a detailed understanding of the underlying pathophysiology.
In recent years, new hypotheses concerning the development of tendinopathy have emerged. One hypothesis suggests that neurochemical substances may be involved in the pathophysiology of tendinopathy. In particular, there is evidence in human tendinosis tissue of local, non-neuronal, production of classic neuronal signal substances by tenocytes.14 This evidence has led to the hypothesis that classic neuronal substances (eg, acetylcholine, catecholamines, substance P, glutamate) may contribute to tendon degeneration, angiogenesis and pain. The roles of these substances in the development of tendinosis are, however, not yet clarified. In upcoming studies, we intend to test this hypothesis using an experimental model.
Experimental models of tendinopathy are not without potential problems.15 When establishing an in-vivo tendinopathy model and interpreting experimental results it is important to recognise that interventions on one side of the body, such as unilateral exercise of a limb, can lead to contralateral changes.16,–,18 For example, experimental manipulations of the rat hind paw of one leg can lead to changes in the other leg.19 20 It has been proposed that contralateral strength improvement is related to peripheral or central (neuronal) adaptations, the main evidence pointing to the latter explanation.21 22
The primary aim of the present study was to establish a reliable in-vivo model for later investigation of nerve-associated substances in the development of tendinopathy. By using contemporary methods for subjective, semiquantitative and quantitative analysis of tendinosis,1 we aimed to confirm and further validate a previously described rabbit model of Achilles tendon tendinopathy.23 Attention was also devoted to the contralateral Achilles tendon to determine whether the contralateral non-exercised tendon in the same animal is an appropriate control.
Materials and methods
Experimental design
An apparatus designed by Backman and collaborators23 24 was used to generate passive dorsiflexion and plantar flexion of the right ankle using pneumatic pistons, which allow the range of motion to be tightly controlled. The optimal range was found to be 9.5 cm, resulting in 20–25° dorsiflexion and 35–30° plantarflexion.23 A band around the pelvis restricted hip and knee motion. The right foot was attached to the piston, while the left leg was not; the pelvis being strapped down prevented ankle movement in the non-exercised leg.
In order to mimic the action of the human Achilles tendon during running, concentric contraction of the triceps surae muscle during plantarflexion was achieved using surface electrodes (pediatric electrodes 40 426A; Hewlett Packard, Andover, Massachusetts, USA). Electrodes were placed 2 cm apart over the triceps surae muscle and were activated by a microswitch in the piston. The microswitch synchronised the stimulation unit (type 14E 10; Disa Elektronik A/S Herlev, Denmark) to cause muscle contraction during the plantarflexion movement of the piston. An impulse of 0.2 ms duration was delivered 85 ms after the initiation of plantarflexion at an amplitude of 35–50 V. The movement frequency was 2.5 Hz (ie, 150 movements per minute). Rabbits underwent training 2 h every second day.
Animals
Twenty-four female New Zealand adult white rabbitswere used. Animals were obtained at a weight of approximately 4 kg (age ranging from 6 to 9 months) and divided randomly into four groups of six. Three groups were exposed to the exercise procedure on their right leg as described above for a total period of 1, 3 or 6 weeks. In between experiments, rabbits were kept in ordinary cages allowing them to move freely. Animals of the fourth group served as controls and underwent no exercise. All rabbits were kept in a laboratory that maintained a 12:12 light–dark cycle.
Animals were anaesthetised during the exercise procedure by intramuscular injection of fentanylfluanison (0.2– 0.3 ml/kg) and diazepam (0.2 ml/kg). Fentanylfluanison (0.1 ml/kg) was injected each 30–45 min to maintain anaesthesia. For analgesia, buprenorphine (0.01–0.05 mg/kg subcutaneous) was given after each 2 h training session. Experiments were approved by the local ethical committee for research on animals.
Sampling, fixation and sectioning
One day after the last exercise, rabbits were killed by pentobarbiterol natrium (60 mg/kg intraperitoneal) and the triceps surae with attached Achilles tendon was collected. From all animals, both right and left Achilles tendons were harvested. Biopsies were taken from three parts of each Achilles tendon: The distal tendon (adjacent to the calcaneus; part a), the midportion of the tendon (b) and the muscle-tendon junction (c). The samples were approximately 5×5 mm.
Some tissue samples were immediately fixed overnight at 4°C in 4% formaldehyde in 0.1 M phosphate buffered saline pH 7.0, then washed in Tyrode's solution containing 10% sucrose at 4°C overnight. Mounting on cardboard in optimal cutting temperature compound (TissueTek; Miles Laboratories, Naperville, Illinois, USA) was performed in transverse orientation, followed by freezing in propane chilled with liquid nitrogen. Samples were stored at −80°C until sectioning. Other tissue samples were mounted and frozen as described above directly after dissection (ie, unfixed). At least one fixed and one unfixed tissue sample was collected from each part of the Achilles tendon from all animals.
Biopsies were sectioned in series at 7 µm thickness using a cryostat. Sections were mounted on chrome-alum gelatine precoated slides and examined under a Zeiss Axioskop 2 plus microscope equipped with epifluorescence optics/filters and an Olympus DP70 digital camera.
Histological evaluation and quantification of tenocytes
Sections from all samples were stained with haematoxylin-eosin for morphological evaluation. Five of the researchers (GA, JES, SF, AS, PD) evaluated the general morphology, including tenocyte morphology and the pattern of collagen organisation, whereas two (GA, JES) made detailed quantifications of tenocytes and one (GA) graded the vascular structures (cf next section).
When quantifying tenocytes, micrographs were taken in three randomly chosen areas of the same slide independently by two researchers. The areas consisted only of tendon tissue proper. This was done using the 40×/0.75 microscope objective (picture size 283×213 µm2).
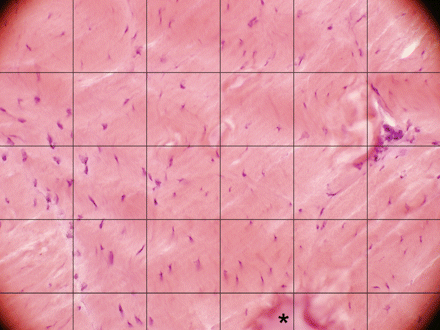
After counting tenocytes, a mean was calculated for the three micrographs. This was repeated for all three parts of the tendon (a, b and c, as described above). To facilitate tenocyte counting, each micrograph was overlaid with a grid (figure 1). Distortions of the tissue were occasionally encountered, so the mean value of undamaged squares was used to compensate for any distorted squares (figure 1).
Section of rabbit Achilles tendon tissue from an animal that had been exercising for 6 weeks, stained with haematoxylin-eosin. The picture represents a micrograph of areas (size 283×213 µm2) used for tenocyte quantification. A grid (of 6×4.5) squares is laid over each picture to facilitate the cell count. Squares containing tissue damaged in the fixation/staining process (asterisk) are given a cell count number representing the average of the cell count in the rest of the squares in the same picture.
Immunofluorescence and evaluation of vascular structures
A mouse monoclonal antibody to CD31 (Dako M0823; Dako, Glostrup, Denmark), which is found on endothelial cells, was used at a concentration of 1:100 on unfixed tissue sections to evaluate vascular structures.25 Briefly, sections were permeabilised for 20 min in 1% Triton X-100 (Merck, Darmstadt, Germany), blocked with 5% normal rabbit serum and 0.1% bovine serum albumin for 15 min, then incubated with the primary antibody overnight at 4°C in a humid environment. After three 5-min washes in phosphate buffered saline and another incubation in normal rabbit serum, sections were incubated with tetramethylrhodamine isothiocyanate-conjugated rabbit anti-mouse IgG (Dako), 1:40, for 30 min at 37°C. Sections were finally mounted in Vectashield H-1000 mounting medium (Vector Laboratories, Burlingame, California, USA).
For the evaluation of CD31 staining, a grading based on a modified Bonar scale26 was used (the Bonar scale in turn being based on previous histological studies).27,–,29 The Bonar scale is used for semiquantitative assessments of histopathological changes in tendinosis, and includes vascularity as one of the four evaluated features, the others being tenocyte appearance, ground substance and collagen.26 In the present study, only vascular grading was applied with some modifications, and each slide was graded based on the general appearance of the vascular network and on the number of vessels, with a higher number of vessels per field giving a higher score. See table 1 and figures 2 and 3 for more detailed information. As a grade of 0–3 was obtained for each part (a, b, c), tendons received a final grade of 0 (normal) to 9 (highly vascular).
(A) Schematic drawing of the rabbit Achilles tendon, transverse section corresponding to the tissue sections investigated in the present study. Larger blood vessels are confined to the loose paratendinous connective tissue in the outer part of the tendon. Branches of these vessels course between larger fibre bundles (area marked b, corresponding to figure 2B). In the deep part of the tendon (tendon tissue proper), small blood vessels course transversely in between collagen bundles and some vessels run in parallel with the collagen fibres (area marked c, corresponding to figure 2C). (B) Transverse section of normal rabbit Achilles tendon stained for the endothelial marker CD31. Tetramethylrhodamine isothiocyanate staining. A blood vessel branch coursing transversely from the loose paratendinous connective tissue in between collagen bundles (corresponds to area marked b in figure 2A) is seen. The two major bundles that are seen correspond to the tendon parts originating from the gastrocnemicus and soleus muscles, respectively. (C) Transverse section of Achilles tendon tissue conforming to tendon tissue proper (corresponding to area marked c in figure 2A), stained for CD31. A transversely coursing blood vessel is seen in between collagen bundles (arrow). If found in small quantities, these vessels are considered to be inconspicuous and normal. A longitudinally coursing blood vessel is also shown (arrowhead). The latter type of blood vessel is increased in tendinosis.27
Transverse sections of rabbit Achilles tendon tissue stained for the endothelial marker CD31; tetramethylrhodamine isothiocyanate stainings. (A) Tissue from an animal in the control (non-exercised) group. Blood vessels are seen in the loose paratendinous connective tissue (asterisk). In the deep parts of the tendon, only occasional, inconspicuous vessels coursing in between collagen bundles (arrow) are seen. The sample is graded 0 in the modified Bonar scale (table 1). (B) Tissue from an animal in the 6-week group (exercised leg). Several conspicuous capillaries occurring in the tendon tissue proper are seen (some indicated with arrows). These run longitudinally in the tendon and correspond to vessels increased in degenerating tendons.27 The sample in b is graded 3 in the modified Bonar scale.
Modified Bonar scale for grading of vascularity in tendinosis
The evaluation was performed by a single observer (GA) who was blinded to the slides' identity. Twenty-five per cent of the slides were randomly re-evaluated to examine the test–retest reliability.
In-situ hybridisation
A custom designed digoxigenin-hyperlabelled oligonucleotide probe (ssDNA) was used to detect rabbit vascular endothelial growth factor (VEGF) messenger RNA (GD1001-DS custom designed; GeneDetect, Auckland, New Zealand)on unfixed tissue sections. In-situ hybridisation (ISH) was performed as previously reported by our laboratory;30this protocol being a variant of an established ISH protocol.31 The probe for rabbit VEGF-mRNA was used at 50 ng in 15 µl of hybridisation solution. The antisense probe sequence was: TGCTGGCCCTGGTGAGGTTTGATCCGCATGATCTGC ATGGTGACGTTG. The corresponding sense digoxigenin-hyperlabelled ssDNA probe was used as a negative control. A control Poly(dT) probe (GD4000-OP; GeneDetect) was used as a positive control. Sections were evaluated by three of the scientists (PD, GA, SF).
Statistics
The Friedman test was used to test for significant differences in tenocyte number and in the vascularity score in different parts of the tendon (a, b, c). To compare tendons of the same rabbit, the Wilcoxon signed rank test was performed. The Kruskal–Wallis test was used to test for differences between groups, followed by pair-wise comparisons using the Mann–Whitney U test, in which p values were multiplied by the number of pairwise tests (Bonferroni correction).
Because a trend was detected regarding tenocyte numbers after 3 weeks of training (see Results section), this was the time point selected for the statistical comparison regarding the vessels. The pairwise Mann–Whitney U test showed no significant difference in vascularity between the control group and the 1-week group, or between the 3-week group and the 6-week group, therefore regrouping was done into the following two groups: ‘less than 3 weeks exercise’, including a control group and 1-week group, and ‘3 weeks exercise or more’, including the 3-week and 6-week groups. For comparison between these two groups, the mean grade of both tendons for each rabbit was used, as there was no significant difference (Wilcoxon signed rank test) between the tendons in any of the groups (see Results section).
Intraclass correlation coefficient (ICC), using a two-way mixed model with a consistency type, was used to check the interrater reliability of the two researchers quantifying the tenocytes. A two-way mixed model of absolute agreement was used for intrarater reliability of vascularity. In both cases the average measures ICC was used. An ICC score of 1 means that there is total agreement between the two examiners/examinations.
A computer program (SPSS 11.0 for Macintosh) was used for all statistical calculations, with significance predetermined at p<0.05.
Results
General morphology
In tendons from the exercised leg of all three test groups (1 week, 3 weeks, 6 weeks), tendinosis-like changes1 were seen to varying degrees. Abnormal tenocyte morphology, irregular collagen and disruption of collagen bundles were prominent in the 3 and 6-week groups (figure 4). It was evident by subjective assessment that the number of tenocytes and blood vessels was increased in the tendons of animals after 3 or 6 weeks of exercise but not after only 1 week. Although there was variability among animals, the most prominent changes were observed in the 6-week group. The 1-week group displayed only very modest changes.
Transverse sections of rabbit Achilles tendon tissue from an animal of the control (non-exercised) group (A) and from an animal that had been exercising for 6 weeks (B), stained with haematoxylin-eosin. In (A) the tenocytes are moderate in number and have an inconspicuous appearance and the collagen is well organised. In (B) the tenocytes are numerous and look abnormal (swollen and irregularly shaped) and the collagen is disorganised and frequently disrupted. A 40×/0.75 microscope objective was used for both images.
Tendons from the non-exercised leg of animals in the three test groups also showed tendinosis-like changes as described above. This was especially evident at 6 weeks. No such changes were noted in the tendons from the control group, which showed a normal appearance of tenocytes, well organised collagen bundles and a modest number of blood vessels coursing between collagen bundles.
Tenocyte number
The Kruskal–Wallis test revealed a significant difference in tenocyte number among groups both for exercised (p<0.001) and non-exercised (p=0.001) legs. The animals in groups that were subjected to exercise for 3 and 6 weeks showed a significant increase in tenocyte number compared with the control group, both in the exercised (figure 5A) and non-exercised (figure 5B) leg (pair-wise Mann–Whitney U test). There was no significant difference in tenocyte number in the 1-week group compared with the control group in either leg (figure 5A,B).
Tenocyte counts. (A) Right leg (exercised at 1, 3 and 6 weeks); (B) left leg (non-exercised at 1, 3 and 6 weeks); (C) right leg versus left leg. (A) Median number of tenocytes in the counted area (size 283×213 µm2) in Achilles tendon tissue from the right leg (exercised leg in test groups) of the animals in the control (non-exercised) group (0) and the test groups (1 week, 3 weeks and 6 weeks). The tenocyte number for 1, 3 and 6 weeks are each compared with the tenocyte number of the control group by pairwise Mann–Whitney U tests (three tests in total). There is a significant increase in tenocyte number in the 3-week group and the 6-week group, compared with the non-exercised group, but no significant (NS) difference in the cell count in the 1-week group, compared with the control group. Error bars indicate interquartile range (IQR). (B) Median number of tenocytes in the counted area (size 283×213 µm2) in Achilles tendon tissue from the left leg (non-exercised leg) of the animals in the control group (0) and the test groups (1 week, 3 weeks and 6 weeks). The tenocyte numbers for 1, 3 and 6 weeks are each compared with the tenocyte number of the control group by pair-wise Mann–Whitney U tests (three tests in total). There is a significant increase in tenocyte number in the 3-week group and the 6-week group, compared with the non-exercised group, but no significant (NS) difference in the cell count in the 1-week group compared with the control group. Error bars indicate IQR. (C) A comparison between the right and left leg for the rabbits of the same group showing no significant differences in tenocyte number between the legs in the control group or between the legs in the 3 and 6-weeks groups (Wilcoxon signed rank test). A slightly lower median cell count (87 cells/area; IQR 78–101) was seen in the right leg of the animals in the 1-week group compared with the left leg (98 cells/area; IQR 91–113). Error bars indicate IQR.
The median number of tenocytes in the counted area (size 283×213 µm2) for the different groups for the right leg was: control group 99 (with the middle 50% of the observations, that is, the interquartile range (IQR), lying between the numbers 93 (Q1=lowest quartile) and 101 (Q3=highest quartile)); 1-week group 87 (IQR 78–101); 3-week group 140 (IQR 132–157); 6-week group 145 (IQR 143–159). The corresponding median number for the left leg was: control group 102 (IQR 99–108); 1-week group 98 (IQR 91–113); 3-week group 144 (IQR 130–160); 6-week group 150 (IQR 139–169).
A Wilcoxon signed rank test showed that there was no significant difference between the right and left leg in the control group nor in the 3-week and 6-week groups (figure 5C). There were slightly fewer cells per area in the right (exercised) leg of the 1-week group compared with the left leg in the same group (p=0.028; figure 5C).
The average measures ICC between the two examiners was 0.810 (95% CI 0.661 to 0.894).
The Friedman test revealed that there was no significant difference in tenocyte number among the different parts of the tendon (a, b, c) in any of the groups.
Vascularity
Qualitatively, a vascular proliferation was noted to occur bilaterally after 3 weeks of exercise. The median vascular grade for the 3 weeks or more group (5.5; IQR 4.5–6.5) was significantly higher than that of the less than 3 weeks group (3.5; IQR 2.6–5.0) (p=0.012; Mann–Whitney U test; figure 6A). There was no significant difference in grade between the legs in any of the four groups (controls, 1 week, 3 weeks, 6 weeks; Wilcoxon signed rank test) or between legs within the less than 3 weeks group or within the 3 weeks or more group (Wilcoxon signed rank test; figure 6B).
Vascular grading. (A) Less than 3 weeks exercise versus 3 weeks of exercise or more; both legs. (B) Comparing right (exercised) and left (non-exercised) legs. Semiquantitative grading of vascular structures according to the modified Bonar scale for vascularity (table 1). Grades 0–3 for three different parts of the tendon (parts a, b and c) were defined (Materials and methods), giving a final grade of 0–9 for each tendon. (A) Median grades for vascularity according to the modified Bonar scale (both legs) are compared for the groups ‘less than 3 weeks exercise’, including control group and 1-week group, and ‘3 weeks exercise or more’, including the 3-week and 6-week groups. The Mann–Whitney U test shows that the median grade for the 3 weeks or more group (5.5; IQR 4.5–6.5) is significantly higher than that for the less than 3 weeks group (3.5; IQR 2.6–5.0). Error bars indicate IQR. (B) Median grades for the right (exercised in test groups) and left (non-exercised) legs are compared for the two groups ‘less than 3 weeks exercise’ and ‘3 weeks exercise or more’. The Wilcoxon signed rank test shows no significant difference (NS) between the legs in either group. Error bars indicate IQR.
The average measures ICC for determining the intrarater reliability was 0.89 (95% CI 0.79 to 0.95) between the original examination and the re-examination.
The Friedman test revealed that there was no difference in the vascular grading between the different parts of a tendon examined (a, b, c) in any of the groups.
Vascular endothelial growth factor mRNA
ISH for the detection of VEGF-mRNA was carried out on tissue from the control group (n=5) and the group exercised for 6 weeks (n=6, exercised leg). There were specific reactions for VEGF-mRNA in some blood vessel walls in all sections when using the antisense probe (figures 7 and 8A). No reactions were seen when using the sense probe (figure 8B). The reactions in the blood vessel walls were most clearly expressed in the tendinosis-like tendon tissue of the 6-week group.
Sections of Achilles tendon tissue from an animal in the 6-week group, processed with in-situ hybridisation (antisense probe), using digoxigenin-alkaline phosphatase detection, for delineating the occurrence of vascular endothelial growth factor (VEGF) mRNA. Blood vessels in a zone of loose connective tissue (endotenon) display specific reactions for VEGF-mRNA (arrows). The asterisk indicates area with tendon tissue proper (collagen bundles).
Sections of Achilles tendon tissue from an animal in the 6-week group, processed with in-situ hybridisation, using digoxigenin-alkaline phosphatase detection, for delineating the occurrence of vascular endothelial growth factor (VEGF) mRNA. Antisense (A) and sense (B) stainings. Groups of cells (arrows and arrowheads) constituting walls of fine blood vessels within the tendon tissue proper are clearly seen in both pictures. The groups of cells in B show only bluish background staining (arrowheads), whereas the group of cells in a shows specific reactions for VEGF mRNA (arrows).
Discussion
The Achilles tendon in this rabbit tendinopathy model exhibited characteristics of human tendinosis, including increased tenocyte number and increased tendon vascularity.1 32 The protocol of this model is thus effective in inducing a tendinosis-like condition in the rabbit Achilles tendon, thereby making the model suitable for further studies on the aspects of tendon pathophysiology. However, the fact that changes were seen also on the contralateral side indicates that one has to be cautious when using this model. That is, the Achilles tendon on the contralateral side is not suitable as a normal control.
Although we can state that the protocol used in this study induced tendinosis-like changes in the exercised Achilles tendon, the study cannot determine what part/parts of the protocol caused these changes. That is, the changes seen in the exercised leg may have been caused by the passive ankle movement, the electrical stimulation, or a combination of both. It can furthermore not be excluded that repetitive delivery of analgesics and/or general anaesthesia contributed to the changes seen. These latter two factors would also be able to influence the contralateral non-exercised (rested) side. There were, in principle, similar Achilles tendon changes seen in the non-exercised side as in the exercised side. However, other possible causes for the contralateral effects should be considered.
Although the reason for contralateral changes in the model cannot be determined here, the phenomenon of bilateral tendinosis is not unheard of. On the contrary, many patients present with bilateral Achilles tendinosis.33 Indeed, 41% of individuals initially presenting with unilateral Achilles tendinopathy developed symptoms on the contralateral side during an 8-year longitudinal study.34 These observations highlight the bilateral characteristics of tendinopathy in the clinical setting.
An attractive, albeit highly speculative, hypothesis is that the changes seen in the non-exercised tendon may be partly induced by the contralateral effects of nerve-related substances in response to the unilateral training. In accordance with this suggestion, it is shown that manipulations involving one of the extremities in the rat, such as the induction of monarthritis19 and injections of the neuropeptide calcitonin gene-related peptide in the hindpaw,20 leads to bilateral changes in levels of neuropeptides in the synovial fluid and oedema formation, respectively. The possibility of bilateral changes in neuropeptide expression and neuronal function in response to unilateral manipulations is of interest given recent observations of nerve-related substances and their receptors in tendon made in our laboratory, including acetylcholine,25 35 catecholamines,36 37 substance P38 and glutamate.39 These nerve-related substances may play an important role in the healing response to trauma affecting various parts of the locomotor system, such as tendons and joints, and may help to explain the frequent progression of unilateral Achilles tendinopathy to bilateral tendinopathy.34 However, this must be extensively investigated in further studies on this rabbit model, before any conclusions regarding the role of nerve-related substances can be determined.
Changes also occur bilaterally at the level of the spinal cord after unilateral manipulations affecting extremities. The induction of monarthritis in one knee joint of the rat thus induces both ipsilateral and contralateral expression of the neuropeptides substance P and calcitonin gene-related peptide in the dorsal horn of the spinal cord.40 In addition, it is well known that the nervous system contributes to the inflammatory component of rheumatoid arthritis and other polyarthrites41 and that this fact explains why rheumatoid arthritis is bilaterally symmetric.41 42 Also, unilateral strength training leads to an increased capacity of the motor cortex to drive the homologous untrained muscles.22 It has been theorised that this is a way for the body to prepare the contralateral side for future exertion of the kind that the exercised/inflamed muscle/joint is enduring. The majority of evidence suggests that modifications in neural functions, at either the central or peripheral level, are responsible for the contralateral increases in strength.43 44 Of interest are recent findings on the effects of unilateral strength training of the wrist of humans. Using transcranial magnetic stimulation experiments, an increased capacity of the motor cortex to drive the muscles in the opposite, untrained, limb was noted.22 This suggests that an increase in ‘cortical’ voluntary drive leads to the effects on the contralateral side.22
Returning to the current experiment, a distinct increase in tenocyte number was detected after 3 and 6 weeks of training, although not after only 1 week of training. Instead, in the 1-week group, there was a tendency towards a decreased number of tenocytes per area in the exercised leg compared with the non-exercised leg. This may be explained by an increase in bound water content seen in exercised tendons,45 thus giving the tendon an increased cross-sectional area. Another explanation may be found in an earlier study on Achilles tendons, in which degenerating tendons were found to have a decreased cell nuclei count in the early stages followed by a marked increase later in the degenerative process.27
In conclusion, this study showed that there were bilateral tendinosis-like changes in the Achilles tendons of rabbits in the current model after 3 or 6 weeks of training. Animals exposed to this protocol are thus suitable for further experimental studies on whether the injection of various signal substances can enhance or reduce the development of tendinosis. The bilateral nature of the changes seen could be a result of central neuronal mechanisms, bilateral expression of nerve-related substances and/or the systemic delivery of anaesthesia and analgesia. In any case, this clearly demonstrates that the contralateral Achilles tendon is an unsuitable control—a fact of great importance to establish before any further studies can be made.
What is already known on this topic
▶ The pathophysiology of human tendinopathy is largely unknown, but the tissue changes seen are of a degenerative-like nature (‘tendinosis’).
▶ Studies suggest the involvement of non-neuronal signal substances in the development of tendinosis.
▶ There is a need for animal models in this research field.
What this study adds
▶ The rabbit Achilles tendon protocol used in this study induced tendinosis-like changes after a minimum of 3 weeks' exercise, which makes it an effective tendinopathy model for future experiments.
▶ The contralateral (non-stimulated) Achilles tendon is not a valid control as tissue changes similar to the exercised side were observed.
Acknowledgments
The authors would like to thank Ms Ulla Hedlund, Mr Adrian Lamouroux and Ms Fellon Robson-Long for excellent technical services and Dr Hans Stenlund for help with the statistics. The authors also thank laboratory technician Mr Fredrik Johansson for initial examinations and antibody testing in the project.
References
Footnotes
-
Funding Financial support was obtained from the Faculty of Medicine at Umeå University (SF, PD), the Sigurd and Elsa Golje Memorial Foundation (PD), the Tore Nilsson Foundation for Medical Research (PD) and the JC Kempe and Seth M Kempe Memorial Foundations, Örnsköldsvik (SF, HA). AS was supported by a travel grant from the Canada–Scandinavia Foundation, as well as a CIHR postdoctoral fellowship. JEG was supported by the Felice Rosemary-Lloyd travel scholarship.
-
Competing interests None.
-
Ethics approval Experiments were approved by the local ethical committee for research on animals.
-
Provenance and peer review Not commissioned; externally peer reviewed.