Article Text
Abstract
Managing tendinopathy in season is a challenge for all sports medicine practitioners. Many of the strategies employed to treat tendinopathy in a rehabilitation setting are not suitable because of the time taken to recover. Management strategies that control pain and maintain performance are required. These include load management, both reducing aggravating loads and introducing pain-relieving loads, medications and adequate monitoring to detect a deteriorating tendon. Other interventions such as intratendinous injection therapies and other direct tendon modalities can be provocative at worst and without effect at best. Research to improve the understanding of management in athletes in season is compromised by ethical considerations and access to willing participants. It is likely to remain an area where clinical advances guide future treatments.
- Tendons
Statistics from Altmetric.com
Tendinopathy (pathology and pain in a tendon) is a prevalent injury in athletes and is very common in the competition season when loads are high. Tendinopathy can prevent full training and competition as athletes who have lower limb tendinopathy have a reduction in the ability to bound, jump, land or change direction that compromises the capacity for dynamic sports performance.
Treating a tendinopathy in season can be very frustrating; the condition is frequently slow to respond to interventions, and it is unrealistic to expect full recovery in season when high loads are continually placed on the tendon. More frustrating is the expectation within sport and sports medicine that tendons should be a quick and easy fix. Sports personnel will allow stress fractures, muscle strains and ligament disruption weeks to recover; yet it may place unreasonably short expectations on recovery time for tendinopathy. Managing pain to acceptable levels, guided by some appreciation of the underlying pathology and tissue properties, underpins the best option for treating tendon pain in season.
The presence of tendon pain is the problem in athletes in season; tendons can have substantial pathology without pain,1 and capacity to load may be unaffected. Pain inhibits the athlete utilising energy storage within the tendon, thereby compromising function and performance. However, tendon structure and pathology must be considered in season. Substantial matrix disorganisation in degenerative pathology may result in areas of the tendon that are less capable of tolerating athletic load,2 and the residual normal tissue within the tendon is subjected to greater load. The only option for repeated failures to accommodate athletic load is a comprehensive rehabilitation programme that can increase the load absorption capacity of the tendon; such a programme is incompatible with ongoing participation in competitive sport.
Features and biology of tendon pain
Tendon pain is well localised with little referral beyond the tendon, unless there is extensive involvement of the associated bursa or fat pad.3 Tendon pain is provoked by loading; the greater the load, the more pain is experienced.4 This pain has a very short latency, experienced only when load is applied and abating quickly on removal of the provocation (eg, hopping). Further, it is unusual for a tendon to trigger pain without load or to be painful at night or at rest, except where it is very reactive or associated with a metabolic or seronegative condition.5
The anatomy, biology and physiology of tendon pain are not fully understood. Contradictory evidence exists on the substances responsible for pain generation, the source of these substances and the pathways of transmission to the central nervous system. Research suggests that abnormal tendon cells produce signalling proteins and the receptors for epinephrine, acetylcholine, glutamate, substance P, tumour necrosis factor α (TNFα) and other neuropeptides.6 Upregulation of these substances can produce a local response driving vascular and tenocyte responses and may also cause a neural response and provoke pain, although this pathway is not fully understood and other mechanisms may complement these tissue changes.7–10
There is also disagreement surrounding an increase in neural ingrowth in patellar tendinopathy. Most of the innervation appears sympathetic rather than sensory, although there can be nerves with combined sensory and sympathetic fibres.11 Increases in receptors for nociceptive substances have been reported such as N-methyl-D-aspartate (NMDA) (glutamate receptor) in patellar tendinopathy12 and neurokinin-1 (NK-1) receptors (substance P) in lateral epicondylalgia13). Similar findings have been reported in the Achilles tendon.8 ,9 ,14 In addition, some degree of central sensitisation has been reported to contribute to the overall perception of pain;15 conversely, a centrally mediated descending inhibition may also be a factor in the resultant pain perceived.16 ,17
Does the continuum model help in an understanding of in-season presentation?
The continuum model of tendinopathy suggests that there are three stages of tendinopathy: reactive, dysrepair and degenerative.18 Multistage pathology may be the most common presentation in season. Overload in an athlete with underlying degenerative tendinopathy will present with a reactive on degenerative tendinopathy. In addition to the high loads, changes in mechanical properties (stiffness, elasticity) at the interface between areas of differing tendon pathology may predispose degenerative tendons to become reactive in the previously unaffected portion of the tendon. Settling the reactive tendinopathy should return the degenerative tendon to its functional, low pain status.
Load and tendon pathology
Tendons that repeatedly store and release energy are vulnerable to developing tendinopathy. It also seems that those athletes who can do this well (jump high, agile athletes) succumb to tendinopathy.19 This may not only be due to their capacity to store large amounts of energy, but also to a genetic component. Brown et al20 showed that better endurance runners were also more inflexible and were more likely to have the TT allele of the Col5A1 gene, previously associated with tendinopathy.
Combined tension and compression (shear) loads at various sites in athletes can also overload the enthesis.21 These loads in association with individual factors, such as genetic predisposition, gender (men are twice as likely to develop tendinopathy), previous load history and biomechanical factors, may also increase the chances of tendinopathy.1 ,22–24
Biologic tissues have a unique capacity to adapt over time to increase load tolerance and energy absorption. This adaptation occurs mechanically (tendon becomes stiffer) and through increased matrix protein production. Type I collagen response to high load in a normal tendon peaks at around 3 days after intense exercise.25 This response to load in appears to be greater in pathological tendons than normal tendons, with a lower resting level and elevated response to loading.26 Grigg et al,27 who investigated the short-term changes in tendon dimensions after exercise, showed that tendons with pain had a smaller change in tendon dimensions after exercise compared to tendons with pathology (no pain) and normal tendons.
Management of in-season tendinopathy
Tendinopathy begins with a mismatch between the tendon's load capacity and load placed on the tendon, most commonly through a sudden and/or substantial change in the load. This can include a return to sport from an (often unrelated) injury or after the off season, where the load capacity of the tendon is reduced due to a loss of a regular high-load stimulus. As tendons respond very slowly to load, a tendinopathic response is triggered if the magnitude or temporal distribution exceeds the tendon's threshold (table 1).
Example of overloads on the Achilles tendon
Load management in season
Assuming reactive tendinopathy underpins in season tendon pain, the key intervention should be to reduce the activation and/or sensitisation of the tenocytes. Removing the stimulus of high loads will ameliorate the tendon cell response given sufficient time. Elastic energy storage increases cell signalling,28 and very high loads may even cause cell death.29 Reducing cell activation may produce a concomitant reduction in cytokine and neuropeptide release and proteoglycan deposition in the matrix, especially the large proteoglycans such as aggrecan and versican. This is a key to preventing further matrix disruption and increased intolerance to load. As well as preventing further matrix destruction, appropriately progressed loads to the tendon will maintain and/or remodel the matrix.30
There are a number of loads that can provoke a reactive tendinopathic response. Consideration of total load on the tendon is an important concept, as a combination of small overloads may induce a reactive response. Care with rapid increase or an excessive tensile load is a major consideration. Similarly, eccentric loading regimes, particularly when superimposed on an already high training load environment, have been demonstrated to increase tendinopathic symptoms and should be avoided in season.31 ,32 Reduction of compressive loads, and especially the combination of compression and tensile loads (tendon shear), is especially important.21 Thus, simple changes such as decreasing the compressive load while maintaining tensile loads may be useful. One effective way to do this is to reduce loads in the outer muscle range, as compression of the tendon against the bone proximal to the enthesis is increased with longer muscle lengths. Reduction of stretching, particularly for entheseal lesions of the Achilles, hamstrings and adductors, can be helpful.33
Compression by itself, such as a direct blow to the tendon or sustained pressure on a tendon, can also provoke tendinopathy.34 A direct blow can induce a marked reactive response that may take weeks to settle, whereas excessive pressure such as a tight bandage, heel counter or sock tends to induce transient tendinopathy that resolves in days after removal of the compression. Friction or firm massage as a treatment to a painful (and likely reactive) tendon can be provocative to the tendons and peritendinous structures,35 ,36 although it has been shown to stimulate protein production in an animal model37 and may be helpful in a more degenerative presentation.
Conversely, loads that reduce pain should be introduced reasonably early. Loading to decrease pain will maintain tendon stimulus, as total or significant removal of tendon load is catabolic for a tendon.38–40 There is literature to support the use of isometric exercise in pain conditions;41 sustained isometric fatiguing muscle contraction recruits segmental and/or extrasegmental descending inhibition mechanisms.42 The recruitment of descending inhibition results in mechanical hypoalgesia and increased pressure pain threshold in healthy humans.41 In reactive tendons, isometric contraction with some load appears efficacious in decreasing pain for several hours. Moderate to heavy loads with machine-based weights rarely provoke pain. These exercises should be completed in the mid to inner range of the muscle-tendon unit to reduce compression. These loads can be repeated several times a day, utilising 40–60 s holds, 4–5 times, to reduce pain and maintain some muscle capacity and tendon load. In highly irritable tendons, a bilateral exercise, shorter holding time and fewer repetitions per day may be indicated.
Provocative tests and objective scoring methods should be used to monitor tendon pain. As the VISA scales score substantially on pain during higher level activity, they are not responsive to change in the short term and are best used on a month-to-month basis. The athlete can monitor tendon response to training loads by completing a simple loading test daily at a similar time (not early morning) (table 2).
Provocative clinical tests useful to monitor tendon pain
Does imaging the tendon help make decisions on in-season management?
Tendons respond to load on a daily basis, well below what we can sense clinically, what the athlete experiences in terms of symptoms and what standard imaging devices can detect. However, waiting for tendon pain as an indication of overload may be a dance with the devil. This is confounded by our knowledge that tendon pathology can exist for years and never actually cause pain, so perhaps it is the magnitude of the response to a load that is important. An instrument that can measure a tendon's response to load would greatly advance the in-season management of tendinopathy.
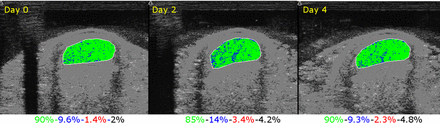
Ultrasound tissue characterisation may offer a way of tendon monitoring in athletes. It uses standard grey scale ultrasound imaging taking 600 axial images (every 0.2 mm over 12 cm) and then reconstructs in the sagittal, coronal plane.43 Using an algorithm, it correlates pixels (reflecting the uniformity of the tissue structure) across a number of contiguous slices and then expresses this as a colour within the tendon (green=good correlation, blue ⩽10% difference in pixels, red ⩾10% difference, black=no correlation). Early research has demonstrated that this imaging approach is valid, reliable and is quite capable of detecting a tissue response to load,44 and clinical studies in elite athletes suggest similar findings (figure 1). Further research is needed to confirm these data.
Changes in echotype after high load imaged with ultrasound tissue characterisation (numbers indicate percentage of green, blue red and black pixels).
Actively reducing tendon pain with medications and injections
There are pharmaceutical means of moderating the reactive tendinopathy that have the potential to ameliorate pain through inhibiting cell activation and proliferation.45 The key elements may consist of inhibition of tenocyte activity through ibuprofen or celecoxib, aggrecan deposition utilising ibuprofen, naproxen or indomethacin46–48 and TNFα. Upregulation of the TNFα signalling system in tendinopathy is present in acute equine tendinopathy,49 and evidence exists for a similar system and involvement in humans.50 Interventions such as doxycycline,45 green tea51 and omega 352 may be considered, although the efficacy appears to be variable, perhaps in keeping with the broader, genetically determined responses to anti-TNFα medication.53
Corticosteroid can act as a significant inhibitor of cell activity and proliferation. This is effective where a quick recovery is needed in a profoundly reactive tendon. A short-pacting corticosteroid (dexamethasone) deposited around a tendon or even orally can be effective,54 and iontophoresis may also be considered, although the delivery is less direct.55
There is considerable literature that suggests that a corticosteroid injection is not indicated for tendinopathy,56 but in the authors’ opinion, this reputation may have been earned through perhaps inappropriate use; in the wrong stage of tendinopathy (degenerative stage), with a long-acting corticosteroid and without satisfactory load management and the subsequent rehabilitation necessary to also rebuild the loading capacity of the tendon.57 No medications or injectables to date have been demonstrated to alter tissue properties; only a tendon load can stimulate remodelling.
Analgesia is often considered to be clinically reasonable and feasible on the grounds that one is treating ‘only pain’. However, pain appears to be biologically important and probably reflects in some way a need to reduce the load stimulus experienced by the tendon cells. Removing pain with analgesic or anaesthetic agents and allowing a player to continue has several issues. First, few oral analgesics are strong enough to control tendon pain; second, injectable anaesthetic agents are often diffused into nearby structures and can affect the athlete's capacity to perform or protect nearby joints; and third, loading a tendinopathic tendon maximally when pain free appears clinically to make it progressively worse. A few ruptures have been reported.58 ,59
Many other injectable medications have been used to treat tendons; unfortunately, they are often used without consideration of the stage of tendinopathy. Many in-season tendinopathies will generally have a reactive aspect and any intratendinous injection therapies in this stage will be provocative, increasing pain in the short term and not reducing pain in the longer term. Postinjection soreness may require lengthy downtime, which causes kinetic chain disuse and catabolism of the tendon (and contralateral tendon). Injections suggested to repair tendons, such as autologous blood and platelet-rich plasma, have little evidence to date to support their use either in season or as part of rehabilitation.60
Some injections reduce pain through a variety of mechanisms; vascular sclerosing injections work mostly as a neurotoxin, reducing pain for several weeks with little effect on the vascular supply.61 Similarly, brisement (injection of saline and a corticosteroid in the peritendon) may have an analgesic effect mostly through the action of corticosteroid but perhaps through neural disruption.62
Surgical intervention
Intratendinous surgery, commonly utilised in recalcitrant cases of tendinopathy, requires a substantial rehabilitation period of around 6–9 months,63 ,64 which effectively excludes it as a consideration for in-season management. More recently, Alfredson65 in the Achilles tendon and Willberg66 in patellar tendon have described a peritendinous operative procedure requiring a rehabilitation period as short as 6 weeks postoperation, enabling this to be a consideration for in-season management. Preliminary long-term results are positive; the removal of plantaris in the Achilles surgery also appears to be effective.67 ,68 This intervention requires careful consideration; the indication for surgery remains, failing a well-constructed rehabilitation programme. Surgery alone, without addressing a musculotendinous and kinetic chain function, does not address the contributing factors and load capacity of the tendon, elements that underpin a good, long-term outcome.
Adjunct treatments in season
Adjunct treatments may be effective to reduce pain, improve function of the musculotendinous unit or the limb or decrease the load on the affected tendon. Therapies that reduce pain include extracorporeal shock wave therapy, which reduces C fibre activity,69 providing some pain relief for several weeks, but there is evidence that it can be disruptive to the tendon tissue structure.70 Supportive strapping or bracing, orthoses, footwear and other equipment choices should also be considered in managing the athlete with in-season tendinopathy as small gains made in this realm may provide enough to allow satisfactory athlete function.
Kinetic chain considerations
Management focus on the presenting tendinopathy is simplistic, as the distribution of absorption of energy across the kinetic chain is an important consideration and each tendinopathy requires a holistic approach to rehabilitation. For example, a restriction of ankle dorsiflexion in a landing has the potential to increase the load on the patellar tendon.24 Other considerations for patellar tendinopathy may include gluteal strengthening and recruitment, calf strengthening and landing re-education, encouraging energy absorption to be distributed across all three major joints or segments. Much of this can be commenced very early in the management cycle, providing symptomatic gains as the presenting tendinopathy is effectively unloaded. Similar approaches are naturally applicable across the other tendinopathies.
How to prevent in-season pain
Early intervention in athletes with tendinopathy is a key element. As with managing stress fractures, early identification, modification of training and return to sport utilising realistic timelines are fundamental to a good outcome. Identification of at-risk athletes, individualising training, monitoring changes in pain and immediate adjustment of loads are essential. Maintaining well distributed functional loads relevant to the sport during the off season in an effort to reduce deconditioning of the muscle-tendon unit and kinetic chain appears to be a key consideration. Further, for athletes who have had significant downtime as a result of surgery or illness, avoiding a rapid return to high tendon load is also essential.
Summary
This clinical perspective has been based on tissue science, pain science, tissue properties and clinical knowledge; however, there is little research in many of these areas. Managing a tendon in season is complex and extends well beyond a simple assessment and exercise sheet, as the clinician needs to stage pathology, determine response to load and determine what loads are affecting the tendon, both extrinsically and intrinsically. Tendinopathic tendons are less tolerant of load changes and management of this is a key element. Complementary interventions may be useful in improving pain and related functions, although they have not been shown to be able to change tendon load capacity. Intratendinous procedures are more likely to produce exacerbation and are not recommended in season where ongoing performance is expected. Peritendinous surgery has promising results within a realistic time period to be considered.
What are the new findings?
-
This paper synthesises research findings and clinical expertise to provide a plan for managing tendinopathy in athletes during the competitive season.
How might it impact on clinical practice in the future?
-
This paper provides a basis for managing athletes with tendinopathy in season.
-
Each athletic presentation will be different, and the principles provided in this paper must be moulded to suit each athlete.
References
Supplementary materials
Rectification
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
Footnotes
-
Contributors Both authors contributed to the development of the concepts and the preparation of the manuscript.
-
Competing interests Prof. Cook has a patent on the use of UTC to detect change in a tendon. A competing interests form has been completed.
-
Provenance and peer review Not commissioned, externally peer reviewed.
Linked Articles
- Rectification