Article Text
Abstract
Objectives: To assess the effects of differing impact exercise protocols on postmenopausal bone loss at the hip and spine.
Design: Systematic review and meta-analysis.
Data sources: Electronic bibliographic databases, key journals and reference lists of reviews and articles.
Review methods: Two independent reviewers assessed controlled trials evaluating effects of impact exercise on lumbar spine, femoral neck and total hip bone mineral density (BMD) in postmenopausal women for inclusion. Heterogeneity amongst trials and publication bias were assessed. Trial quality assessment was also performed.
Results: Impact protocols that included jogging mixed with walking and stair climbing, and protocols that incorporated impact exercise with high-magnitude loading (resistance exercises), were effective at lumbar spine (weighted mean difference (random effects) 0.025 g/cm2 95% CI (0.004 to 0.046) and 0.016 g/cm2 95% CI (0.005 to 0.027); p = 0.02 and p = 0.005 respectively), although heterogeneity was evident (I2 = 88% and I2 = 73%, where I2 measures the extent of inconsistency among the trials). Effects on femoral neck BMD following these types of protocols were significant (weighted mean difference (fixed effect) 0.022 g/cm2 95% CI (0.014 to 0.030); p<0.001 and 0.005 g/cm2 95% CI (0.001 to 0.010); p = 0.03 respectively). High-impact only and odd-impact only protocols were ineffective in increasing BMD at any site.
Conclusion: Mixed loading exercise programmes combining jogging with other low-impact loading activity and programmes mixing impact activity with high-magnitude exercise as resistance training appear effective in reducing postmenopausal bone loss at the hip and spine. Other forms of impact exercise appear less effective at preserving BMD in this population. However, diverse methodological and reporting discrepancies are evident in current published trials.
Statistics from Altmetric.com
Osteoporosis is a serious, disabling condition that is commonly seen in clinical practice. The condition increases the risk of fractures in later life, with one in two women and one in five men suffering a fracture after the age of 50.1
Weight-bearing exercise has been promoted for prevention and management of postmenopausal osteoporosis.2 3 4 However, current guidelines are equivocal regarding the best mode of weight-bearing exercise for optimum bone augmentation in postmenopausal women. Recommendations have included either: avoidance of high-impact activity altogether5; integrating both high and low-impact activities4; or simply engaging in “weight-bearing” activity without any advice regarding the degree of impact.3
Whilst walking may be a feasible form of exercise for postmenopausal women that confers a number of health benefits,6 its osteogenic affect on bone density is questionable.7 The American College of Sports Medicine (ACSM) position stand on physical activity and bone health recommends regular weight-bearing endurance activities, including jogging and jumping, for preserving bone mass in adulthood. However, the ACSM position stand also concludes that it is not currently possible to quantify exercise intensity in terms of bone loading forces and that menopause related bone loss may not be attenuated by vigorous activity.8
Early meta-analyses of exercise effects on bone mass in postmenopausal women, synthesising high and low impact protocols together, have observed varying effects at the hip9 10 11 and spine.10 11 12 A more recent Cochrane review,13 also synthesising high and low impact protocols together along with resistance training, found that weight-bearing exercises were effective on bone mineral density at the spine.
The purpose of the present systematic review and meta-analysis is to give specific consideration to the skeletal loading characteristics of different impact exercise interventions evaluated in randomised and non-randomised controlled trials and to evaluate the differing effects on hip and spine BMD in postmenopausal women.
Methods
We carried out our meta-analysis in line with Cochrane Collaboration recommendations and quality of reporting of meta-analyses guidelines,14 15 along with the recommendations made by the Cochrane Musculoskeletal Group for improvements in systematic reviews of therapies for musculoskeletal conditions.16
Systematic searches of the following databases from their inception to end March 2008 were undertaken: MEDLINE (1966), EMBASE (1980), PubMed (1966), Web of Science (1945), Sports Discus (1975), EBMZ (1917), and ProQuest (1995). Text words, key words and subject headings used in the searches included: women or females; running, exercise, physical therapy or physical activity; bone density, bone mineral density or bone mass; osteoporosis or osteopenia; clinical trial, controlled trial or randomised controlled trial. Additional references from 1986 to end March 2008 were searched for manually in selected peer-reviewed journals (Bone, Calcified tissue international, Journal of bone and mineral metabolism, Journal of bone and mineral research, Osteoporosis international and Medicine and science in sports and exercise); along with reference lists of other exercise reviews in the area,9 10 11 12 13 reference lists of articles identified for inclusion and web searches (http://scholar.google.co.uk/). Citations were entered into reference management software, Reference Manager V.11 (Thomson ResearchSoft, Carlsbad, CA, USA).
Trials reported as peer-reviewed articles, abstracts, theses and dissertations were eligible for inclusion, as were trials published in languages other than English. Only study groups from controlled trials of impact exercise interventions were included. Where publications were by the same institution, group or author, clarity was sought regarding whether BMD data from the same study population had been reported in more than one publication. Where trials reported BMD data for the same participants in more than one publication, data from only one of the publications were included to avoid double counting participants in the meta-analysis.17
Participants were defined as sedentary postmenopausal women. Trials recruiting female samples drawn from active populations such as aerobics or fitness classes, where existing exercise levels could already have had an effect on BMD, were excluded. Treatment groups comprising men only were excluded as were treatment groups including both men and women where data for the women only could not be obtained.
The interventions of interest were any exercise protocol that included any ground reaction force generating impact activity such as running or jumping-type movements where both feet leave contact with the ground. Treatment groups investigating the effects of impact activity combined with other forms of skeletal loading exercise such as resistance training were also included.
Outcome measures for this review were defined as BMD (BMD g/cm−2) at the lumbar spine, femoral neck and total hip measured by radiographic techniques (single photon absorptiometry, dual photon absorptiometry (DPA) or dual x ray absorptiometry) with standard deviations.
Data were extracted from each article independently by two reviewers (MMSJ and SC). Details extracted included: participant characteristics, numbers of allocated participants and numbers of participants followed-up, length of treatment, attrition, compliance, exercise supervision, any adjuvant pharmacological or nutritional therapy affecting bone that participants were either already taking or had been prescribed to them as part of the intervention, region of interest (ROI) assessed and scanning technique and BMD values with standard deviations.
Absolute change values in BMD from baseline to follow-up were used in the analyses. Where these values were not available from the original article or author, these were calculated using baseline and follow-up values. Standard deviations for these change values were then imputed using a correlation coefficient value (r), calculated using baseline, follow-up and change values, where presented in other included studies. The method is detailed in the Cochrane reviewers’ handbook.14
In order to include trials with more than one treatment group (for example, jumping and jumping combined with resistance training treatment arms) but only one control group, each treatment group was included separately within the meta-analysis, but with the control group participant number divided out equally between the comparisons. This process ensures that control participants are not counted more than once within the meta-analysis.17
Given that BMD values are continuous data, the weighted mean difference (WMD) method was used for combining study effect size estimates. In this method the pooled effect estimate represents a weighted average of all included study group comparisons. Weighting assigned to each individual study group comparison result in the analysis is in inverse proportion to the variance. This method assigns more weight in the meta-analysis to larger trials and less weight to the smaller ones.18 WMDs were calculated using fixed-effect and random-effects models.
Heterogeneity of net study group changes in BMD was examined using the Q statistic. Cochran’s Q statistic is computed by summing the squared deviations of each trial’s estimate from the overall meta-analytic estimate, weighting each trial’s contribution in the same manner as in the meta-analysis. p Values are obtained by comparing the statistic with a χ2 distribution with k-1 degrees of freedom (where k is the number of trials). A p value of <0.10 was adopted since the Q statistic tends to suffer from low differential power.19 The formal Q statistic was used in conjunction with recently proposed methods (I2) for assessing heterogeneity.19 The statistic I2 measures the extent of inconsistency among the trials’ results, interpreted as approximately the proportion of total variation in trial estimates that is due to heterogeneity rather than sampling error.19
Effect sizes with a corresponding I2 value of ⩽50% were considered to have low heterogeneity, those with 50–75% were considered moderate and I2 values of ⩾75% were considered highly heterogeneous.19 A random-effects model was used to analyse results which were determined to be heterogeneous (I2>50%).20 Both fixed-effect and random-effects outcomes for all analyses are reported. Tests for overall effect (Z score) were considered significant at p<0.05. Subgroup analyses were defined a priori to investigate hypothesised differences in the magnitude of treatment effects across trials due to variations in the skeletal loading characteristics of the protocols.21 22 Exercise protocols were categorised according to the impact classifications described by Nikander et al21 and acceleration forces observed by Vainionpää et al.22 Categories were: high-impact loading, such as vertical jumps, rope jumping or running at >9 km/h; odd-impact loading, such as aerobic or step classes, bounding exercises, agility exercises and games where movements included directional elements that the body is not normally accustomed to; low-impact loading such as jogging at <9 km/h; and combined loading studies of impact activity mixed with high-magnitude joint reaction force loading through resistance training. Sensitivity analyses were also undertaken to assess aspects of trial design, specifically randomisation.
Publication bias was examined through funnel plot inspection.17 Funnel plots provide a scatter plot of the treatment effects of included trials against a measure of the trial’s sample size. In the absence of bias the plot should resemble an inverted symmetrical funnel. Visual inspection of funnel plots provides a generic and accepted method to assess publication bias in meta-analysis.17
Meta-analysis and production of all graphics was performed using STATA V.10 (StataCorp, TX, USA).
An assessment of trial quality was undertaken for comparative purposes using the questionnaire described by Jadad et al.23 This is a three-item instrument that provides an assessment of bias, specifically randomisation, blinding and withdrawals/dropout. All questions are designed to elicit yes (1 point) or no (0 point) answers. The total number of points available ranges from 0 to 5. The instrument awards a maximum of 2 points for randomisation, a maximum of 2 points for blinding and a maximum of 1 point for withdrawals/dropout.
Results
From the searches, 122 exercise trials were identified for potential inclusion and full text versions obtained (fig 1). Twenty-seven of the trials evaluated impact exercise interventions. Twelve impact trials did not meet all inclusion criteria for this review and were excluded, reasons for exclusion are given in fig 1 (details of all excluded studies are available from the author). Fifteen trials comparing impact exercise with a non-exercise control group and reporting BMD outcomes assessed by radiographic techniques at the hip and/or spine in postmenopausal women were included (table 1).24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 Study group allocation was reported as randomised in ten of the included trials.24 25 26 27 28 33 34 35 36 38
Flow diagram of trial selection process.
Details of controlled trials of walking effects on bone mineral density
Participant characteristics
With the exception of one trial that recruited Japanese participants,37 all included trials recruited Caucasian women. Where reported, years post menopause was variable ranging from approximately 1–14 years. Median years post menopause was 5.2 years. One trial recruited both post- and perimenopausal women.27
Pharmacological therapy use
Seven of the trials either excluded hormone replacement therapy (HRT) use among participants, or reported that none of the included participants were taking it28 29 30 31 32 37 38 (table 1). Five trials recruited both HRT users and non-users.26 27 34 35 36 One trial recruited both HRT users and non-HRT users who were assigned by HRT usage to exercise or control (four groups)25 (table 1). Three trials recruited non-HRT users and were factorially designed to compare exercise effects with or without adjuvant HRT versus control.30 31 32 One trial was similarly designed to compare exercise effects with bisphosphonate therapy or placebo.38
Exercise protocols
Two trials evaluated exercise protocols of high-impact loading where vertical jumping or skipping was the singular mode of exercise.24 37 Five trials evaluated group exercise interventions providing odd-impact loading forces.26 28 33 34 38 Four trials evaluated combined loading protocols as either circuit-type or other group exercise activity where odd-impact and/or high-impact exercises were combined with resistance training and/or weighted vest work.25 28 29 35 Four trials evaluated the effects of low-impact protocols where jogging was combined with other low impact activity on a saggital plane in the form walking and stair-climbing (table 1).27 30 31 32
Supervision and compliance
Details regarding supervision of the exercise sessions was reported in 14 of the trials,24 25 26 27 28 29 30 31 32 33 34 35 38 of which nine reported that all exercise sessions were supervised24 25 26 28 30 31 32 35 38 (table 1). Compliance with the prescribed interventions as a percentage of sessions attended was reported on in 10 trials,24 25 26 27 28 29 33 34 35 38 where it ranged from 50% to 91% (table 1).
Bone mineral density assessment
Duration of the included trials was variable with final BMD assessment ranging from six months to five years. Median duration was 12 months. One trial reported results at a 5-year follow-up36 to another included trial.35 Femoral neck BMD was reported in the initial trial,35 whereas total hip BMD was reported at the 5-year follow-up (table 1).36
BMD at lumbar spine was assessed in 11 trials,24 25 26 27 29 30 31 32 34 37 38 and femoral neck BMD was assessed in 1324 25 27 28 29 30 31 32 33 34 35 37 38 (table 1). Total hip BMD was assessed in four of the trials,29 34 36 37 however the number of study group comparisons did not permit any subgroup analyses by exercise category for this ROI.
Absolute change values with SD were available for seven trials,24 25 28 29 34 37 38 and imputed for eight.26 27 30 31 32 33 35 36 Table 2 summarises all meta-analysis comparisons undertaken.
Summary of meta-analyses, sensitivity and subgroup analyses by region of interest
Loss to follow-up
Loss to follow-up (participants assigned to study groups versus those analysed at end-point assessment) was reported in seven trials25 26 27 28 29 30 33 38 (table 1).
Quality assessment score
The quality assessment instrument scores awarded to trials ranged from 0–3 (table 1) on a scale allowing values of 0–5.23 All RCTs were allocated one point for being described as randomised, with only one RCT allocated an extra point for including a description of an appropriate randomisation method.28 No trial gained points for blinding of participants or contained a description of adequate concealment of allocation. Eight of the included trials were allocated one point for a description of withdrawals.25 26 27 28 29 31 33 38 Only one of the included trials acquired a total quality score of three28 and three of the trials acquired no points at all.30 32 37
Meta-analysis
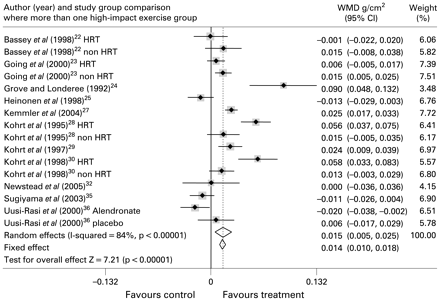
Eleven of the included trials assessed lumbar spine BMD and provided 16 study group comparisons of exercise interventions versus control. A total of 442 participants were assigned to exercise and 250 to non-exercise control. Initial meta-analysis including all study groups was highly heterogeneous (I2 = 84%) for effects of impact exercise interventions on BMD at this site. The combined weighted mean difference (WMD) in BMD was 0.015 g/cm2 (WMD (random effects) 95% CI, 0.005 to 0.025; p = 0.004)).
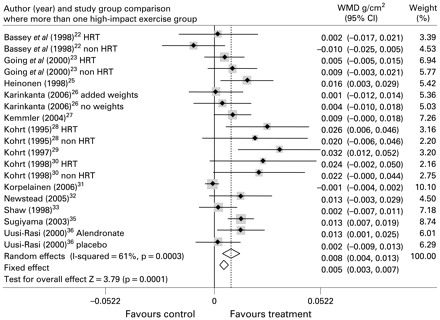
The 13 trials that assessed femoral neck BMD provided 19 study group comparisons totalling 606 treatment participants and 459 controls. Heterogeneity of study effects was observed in this analysis (I2 = 61%). Amongst these study groups, impact exercise resulted in a smaller increase in BMD at this site of 0.008 g/cm2 (WMD (random effects) 95% CI (0.004 to 0.013); p = 0.001).
Four trials assessed total hip BMD and provided 4 study group comparisons including a total of 87 participants who served as treatment participants and 70 who served as control. Results from this analysis were highly heterogeneous (I2 = 91%). Impact exercise resulted in an increase in BMD at this site of 0.013 g/cm2 (WMD (random effects) 95% CI, 0.001 to 0.024; p = 0.37)).
Figures 2 to 4 show the results from meta-analysis of all included trials. Table 2 lists results from all sensitivity and subgroup analyses.
Forest plot for inclusion of all included trial study groups assessing lumbar spine BMD. Diamonds represent overall weighted mean difference (WMD g/cm2) for random and fixed effect models with 95% CI.
Forest plot for inclusion of all included trial study groups assessing femoral neck BMD. Diamonds represent overall weighted mean difference (WMD g/cm2) for random and fixed effect models with 95% CI.
Forest plot for inclusion of all included trial study groups assessing total hip BMD. Diamonds represent overall weighted mean difference (WMD g/cm2) for random and fixed effect models with 95% CI.
Sensitivity analysis including only trials of random design (RCTs), generally did not show any divergence from the heterogeneity evident in the analyses including all trials at lumbar spine. Nine RCT study group comparisons assessing lumbar spine BMD24 25 26 27 34 38 were highly heterogeneous (I2 = 76%). The WMD in BMD at this site was 0.006 g/cm2 ((random effects) 95% CI , −0.006 to 0.019; p = 0.324). However, low heterogeneity was observed amongst the 12 RCT study groups assessing femoral neck BMD24 25 27 28 33 34 35 38 (I2 = 34%). The WMD in BMD amongst RCT study groups at this site was 0.002 g/cm2 ((fixed effect) 95% CI, −0.001 to 0.004; p = 0.141).
The subgroup analysis of trials evaluating protocols of high-impact exercise was consistent in showing no positive effects of this type of exercise on BMD at the lumbar spine (I2 = 43%). The femoral neck analysis for this type of exercise was heterogeneous (I2 = 75%). The observed effects of this type of exercise on BMD were non-significant at both sites. Non-significant treatment effects at both lumbar spine and femoral neck were also confirmed in the subgroup analyses of protocols evaluating odd-impact exercise protocols.
Subgroup analyses for the effects of combined loading protocols that incorporated impact activity with high-intensity resistance exercises were heterogeneous in having a positive effect at the lumbar spine (I2 = 73%). An increase in BMD of 0.016 g/cm2 ((random effects) 95% CI, 0.005 to 0.027; p = 0.005) was observed at this site. A small, but statistically significant positive effect on femoral neck BMD was also observed following this type of exercise regime (I2 = 0%; WMD 0.005 g/cm2 (fixed effect) 95% CI, 0.001 to 0.010; p = 0.027).
A significant effect on femoral neck BMD was also observed following low-impact protocols that included jogging mixed with walking and stair climbing (I2 = 0%; WMD 0.022 g/cm2 (fixed effect) 95% CI, 0.014 to 0.030; p<0.001). Effects at lumbar spine were highly heterogeneous (I2 = 88%) in showing an increase of 0.021 g/cm2 ((random effect) 95% CI, 0.014 to 0.028; p<0.001) following this type of exercise.
Funnel plots were produced for the effects of impact exercise interventions on lumbar spine BMD from all included trials (fig 5). Similar plots were also produced for femoral neck outcomes (fig 6). Visual inspection of these plots indicated some degree of asymmetry of trials showing a negative treatment effect at lumbar spine, whereas the femoral neck displayed greater symmetry of both negative and positive treatment effects.
Funnel plot for inclusion of all included trial study group comparisons assessing lumbar spine bone mineral density.
Funnel plot for inclusion of all included trial study group comparisons assessing femoral neck bone mineral density.
Discussion
The primary purpose of this study was firstly to undertake a systematic review and meta-analysis of trials assessing the effects of different modes of impact exercise on bone mineral density (BMD) at the hip and spine in postmenopausal women. The second purpose was to help provide more information on types of impact exercise for the purpose of prescribing optimal exercise regimes for postmenopausal bone loss.
Our findings indicate that structured exercise programmes that combine differing types of impact loading with high-magnitude loading (resistance training) and protocols that combine jogging with other forms of low-impact exercise along the saggital plane of movement such as stair climbing and walking, have the potential for preserving BMD at the lumbar spine and femoral neck in postmenopausal women.
We included both randomised and non-randomised trials reported in peer-reviewed journals, dissertations or abstracts. In concordance with Altman et al,39 our analyses including only RCT data yielded more conservative effect estimates compared with the included trials that employed non-random allocation methods (meta-analysis not presented). In one of the earliest meta-analyses of exercise effects on bone in women, Wolff et al11 also observed that RCTs showed a modest effect at both lumbar spine and femoral neck. In our analyses combining all included impact trials, positive effect estimates at both lumbar spine and femoral neck were evident. Restricting the overall analyses to only RCT study groups resulted in non-significant findings. We were unable to restrict our subgroup analyses by exercise category to RCTs due to a limited number of trials.
The Cochrane review of Bonaiuti et al13 included only RCTs evaluating exercise effects on BMD. Their subgroup analyses by exercise type demonstrated significant effects at lumbar spine following aerobic or weight-bearing activity. However, their analysis combined diverse treatment groups in terms of the loading characteristics of the protocols including: aerobic classes,26 walking,40 41 bench stepping,42 co-ordination and balance classes43 and high-intensity resistance training.44 45 46 Similarly, Kelley,47 in a meta-analysis of exercise effects on regional bone density including subgroup analyses differentiating between aerobic training and strength training, combined not only both impact and non-impact protocols in the aerobic exercise analysis, but also reported a combined effect size for lumbar spine and femoral neck. In a subsequent meta-analysis of aerobic exercise effects on lumbar spine, Kelley observed significant positive effects.12 However, the included trials were diverse in their loading characteristics and the analysis combined impact exercise,26 40 41 42 48 49 50 51 with non-impact exercise.52 53 Three walking protocols were included in the analysis.40 41 51 In our recent meta-analysis of walking only effects of postmenopausal bone loss, we observed no significant effects on BMD at this site.7 Kelley’s meta-analysis of aerobic exercise effects at the hip9 was also similar to his spine review,12 in terms of the combination of both impact and non-impact trials included in the analyses.30 42 48 51 52 54 A significantly positive overall effect size was observed, but from combining all hip ROI BMD outcomes (18 in total) reported in the included trials. We restricted our hip analyses to femoral neck BMD and total hip BMD, analysed separately. The femoral neck site is widely recognised to be related to substantial osteoporotic fracture,55 with total hip considered the best predictor of any hip fracture and that includes the femoral neck and trochanter.56 However, we were unable to perform any subgroup analysis by exercise for the total hip ROI.
To our knowledge, the present study is the first systematic review to include a meta-analysis that attempts to not only assess the effects of impact exercise on BMD outcomes in postmenopausal women, but to also try and identify the optimum impact exercise programme to best preserve BMD in this population. In a review of factors interacting with physical activity affects on bone in postmenopausal women, Borer57 reports that increases in BMD in this population following exercise have usually been modest and asks whether principles shown to produce increments in bone mass using animal models have been appropriately applied to human studies. These are namely that: adaptive bone response requires dynamic rather than static mechanical stimulation; adaptive bone response requires supra-threshold intensity; osteogenic response is proportional to strain frequency; adaptive bone response is improved with brief but intermittent exercise; and adaptive bone responses require an unusual pattern of bone loading. The largest effect sizes we observed at both lumbar spine and femoral neck were from protocols that combined jogging with walking and stair climbing,27 30 31 32 whilst protocols of odd and high-impact loading combined with high-magnitude loading (high-intensity resistance training), were highly effective at lumbar spine only.25 29 This would suggest that these specific exercise formats provide a loading stimulus that is both adequate in its intensity, and novel in its loading pattern at these sites. However, because of the differing combinations of skeletal loading activities evaluated in the trials included in these analyses, it is unreasonable to recommend exercise programmes based on only the impact components of these regimes alone (jogging, odd-impact, high-impact).
In addition to our systematic review and meta-analysis we also assessed aspects of trial quality of our included trials using a widely utilised instrument. However, points for blinding awarded by the instrument were redundant for any of the included trials reflecting a limitation of this instrument when assessing quality of exercise trials. We did not perform any analyses by trial quality score as aspects of trial design, blinding and attrition may have been more influenced by the level of reporting of these aspects in our included trials. The majority of our trials were published prior to the CONSORT (Consolidated Standards of Reporting Trials) statement.58 Because aspects of study quality were not reported in our included trials, does not mean that they were not undertaken.
Examination of funnel plots revealed some asymmetry of negative effect sizes for both lumbar spine and femoral neck BMD outcomes. Notable positive effects on both plots were the study groups also taking hormone therapy from two separate trials by Kohrt.30 32 These 9-month programmes included walking, jogging and stair climbing for 45 minutes, three times a week, with an emphasis on alternating bursts if higher intensity effort, although subjects were allowed to self-select which could have introduced bias. Furthermore, the additive effects of hormone therapy combined with the exercise, may have also contributed to the positive findings from these trials.59 Our subgroup analysis for the exercise category including the trials by Kohrt et al30 32 also included the trial by Heinonen et al27 that recruited both peri- and postmenopausal women. Analysis excluding this trial (not presented) did not significantly alter the effect sizes we observed for this mode of impact exercise.
The primary outcome for this review was BMD which is a surrogate marker for fractures.45 A prospective cohort study has found that walking for at least 4 h/wk was associated with a 41% lower risk of hip fracture compared with walking for less than 1 h/wk. Any similar analysis of other impact activity is, to date, unavailable. Furthermore, we were unable to assess reduction of fracture risk as an outcome in the present study.
The findings from our review and meta-analysis are limited by trials recruiting highly selected samples of women of varying ages. In addition, the trials were variable in terms of study design, randomisation methods and treatment protocols. Trials of complex interventions such as exercise continue to present methodological challenges for meta-analysis. Nonetheless, where reported, compliance with the exercise protocols was good among our included trials and very few adverse events associated with the protocols were reported. Of note, a number of the exercise interventions were progressive in order to allow participants to become accustomed to the impact nature of the exercises.
Current recommendations regarding optimum exercise for preserving bone mineral density in postmenopausal women require revision. Interventions that include exercises that provide adequate skeletal loading and that are directly targeted at specific skeletal regions should be recommended and clearly described.
What already know on this topic
The benefits of weight-bearing exercise as therapy for preserving bone density in postmenopausal women are unclear. Methodological differences in previous reviews of aerobic type exercise, notably in relation to the skeletal loading properties of protocols of included studies, have contributed to therapeutic uncertainty regarding prescribing impact exercise to preserve bone mineral density in postmenopausal women.
What this study adds
Our systematic review and meta-analysis suggests that impact exercise in the form of jogging when combined with other low-impact activity, such as stair climbing and walking, and programmes combining impact exercise with high-magnitude exercise in the form of resistance training have a positive effect on preserving bone mineral density in postmenopausal women. Guidelines regarding the types of weight-bearing activity for preserving postmenopausal bone density require revision. Where appropriate, specific modes of impact activity need to be integrated into well-designed and safe exercise programmes for postmenopausal women.
REFERENCES
Footnotes
Competing interests None.